Preparation method of diterpene-based antibiotic for livestock and poultry
A technology for generating formulas and compounds, which is applied in the field of preparation of diterpene-based antibiotics for livestock and poultry, can solve problems such as the impact of industrial production of compounds and the reduction in the conversion rate of target products, and achieve stable properties, less impurities, and simple preparation processes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
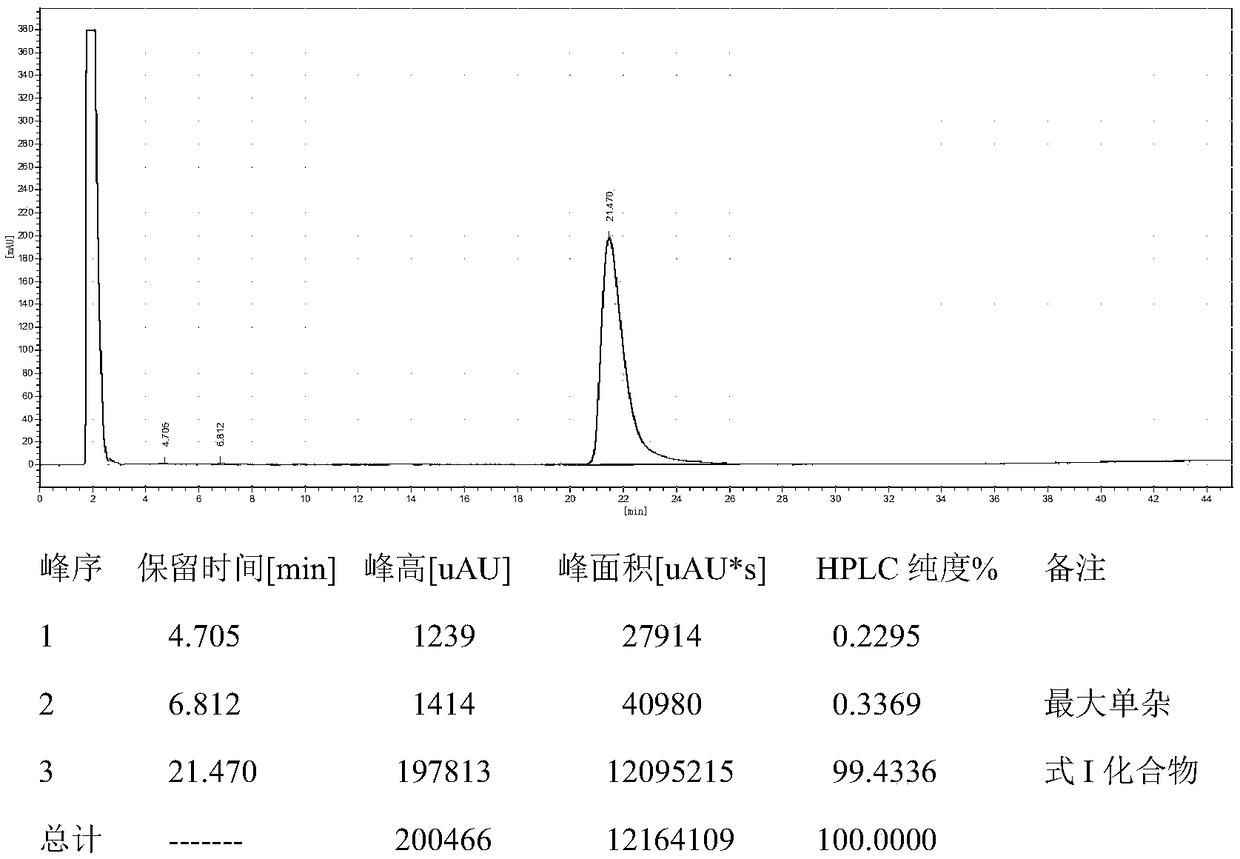
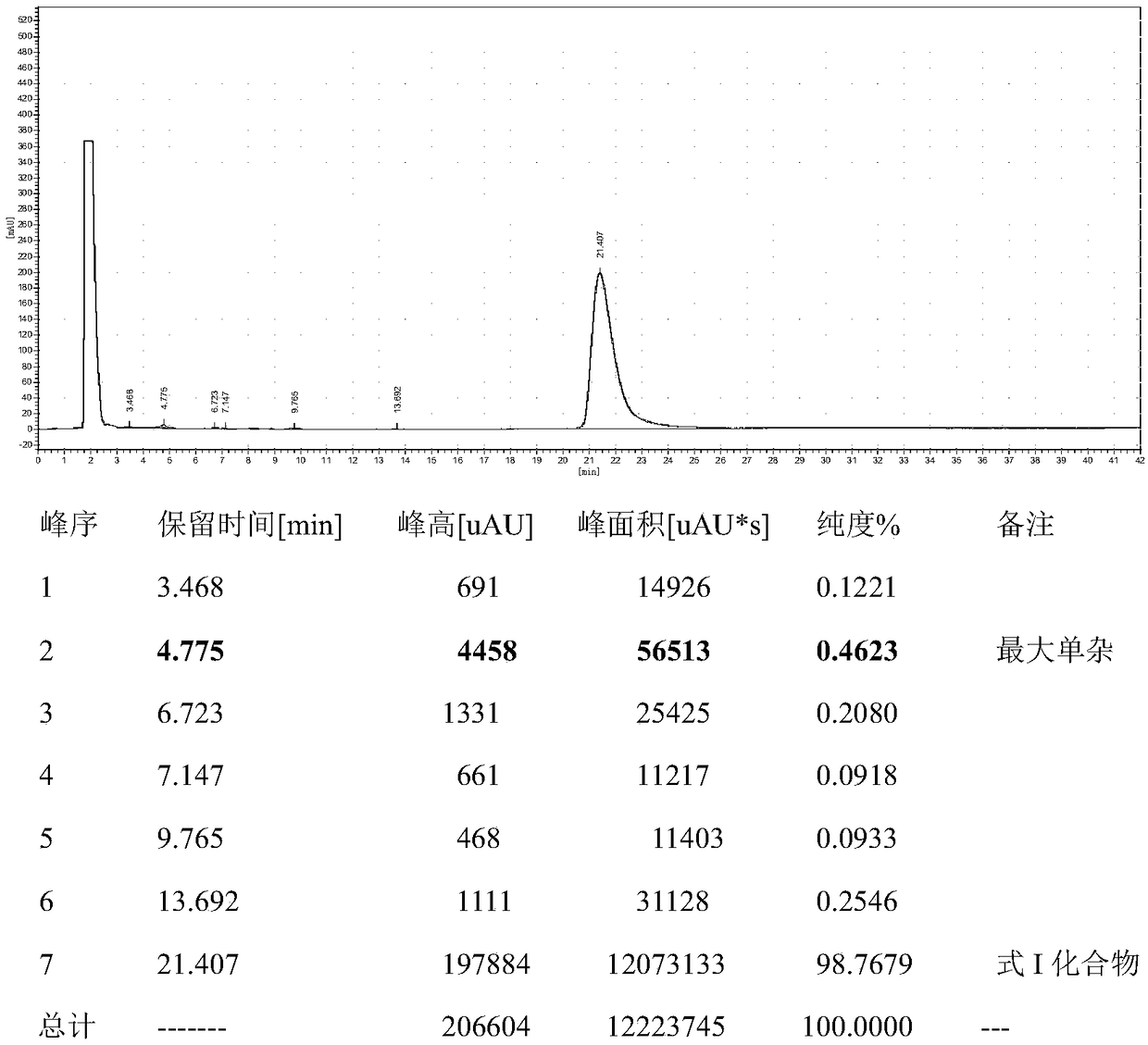
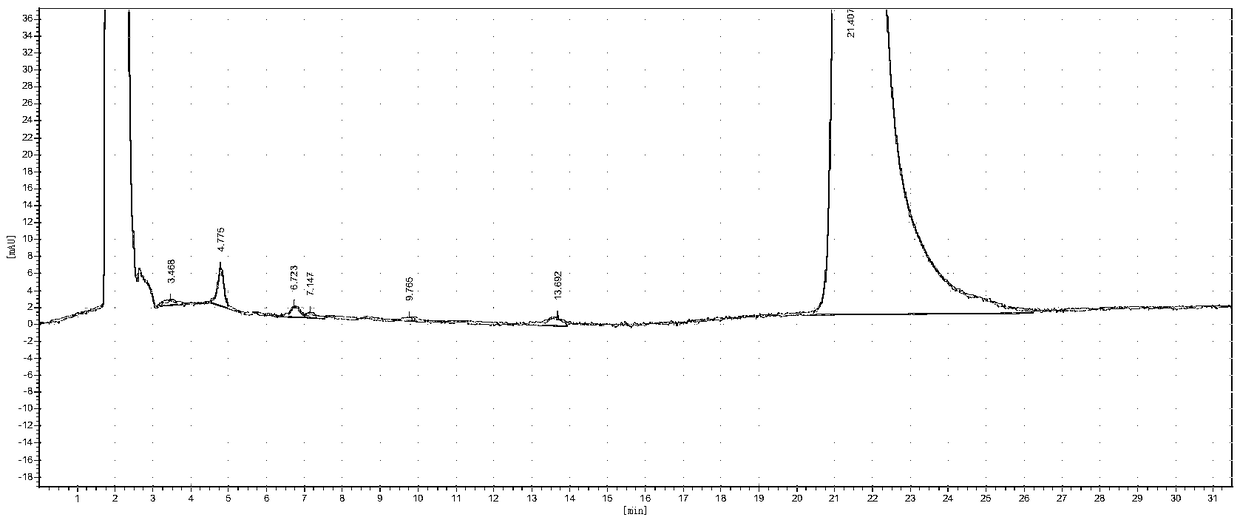
[0041] Add 120mL of toluene and 60mL of water into a 500mL three-necked flask, then add 14.16g (0.062mol) of diethylaminoethylthiobenzoate, 2.604g of lithium hydroxide monohydrate, and 3.22g of tetrabutylammonium hydroxide, stir for 30min, and slowly 30 g (0.062 mol) of pleuromutilin benzoate was added, and after the addition was complete, the temperature was raised to 75°C-80°C, and the reaction was kept for 6 hours, and the reaction was completed. The sample was quantified by HPLC, and 29.91 g of the compound represented by formula I was obtained, with a conversion rate of 94.6%. HPLC purity 98.5558%, see the spectrum and data Figure 4 and 5 .
Embodiment 2
[0043] Add 120mL of toluene and 60mL of water into a 500mL three-necked flask, then add 18.67g (0.062mol) of diethylaminoethylthio p-toluenesulfonate, 6.25g of liquid caustic soda (NaOH), 0.25g of tetrabutylammonium chloride, and stir for 20min , slowly added 30 g (0.062 mol) of pleuromutilin benzoate, after the addition was complete, the temperature was raised to 80° C.-85° C., and the reaction was kept for 7.5 hours, and the reaction was completed. The sample was quantified by HPLC, and the compound represented by formula I was obtained, and the conversion rate was 93.6%. HPLC purity 98.8715%.
Embodiment 3
[0045] Add 120mL of toluene and 60mL of water into a 500mL three-necked flask, then add 18.36g (0.062mol) of diethylaminoethylthio-p-nitrobenzoate and 6.25g of liquid caustic soda, stir for 10min, and slowly add pleuromutilin benzoic acid 30 g (0.062 mol) of ester, after the feeding is completed, the temperature is raised to 75°C-85°C, and the reaction is kept for 6.5 hours, and the reaction is completed. Sampling was carried out by HPLC for quantification, and 29.66 g of the compound represented by formula I was obtained, with a conversion rate of 93.8%. HPLC purity 99.1115%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com