Fusion protein of thrombopoietin and preparation method and application thereof
A thrombopoietin and fusion protein technology, applied in the field of genetic engineering pharmaceuticals, can solve the problems of unpredictable results and immaturity, and achieve the effect of promoting platelet production and long half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
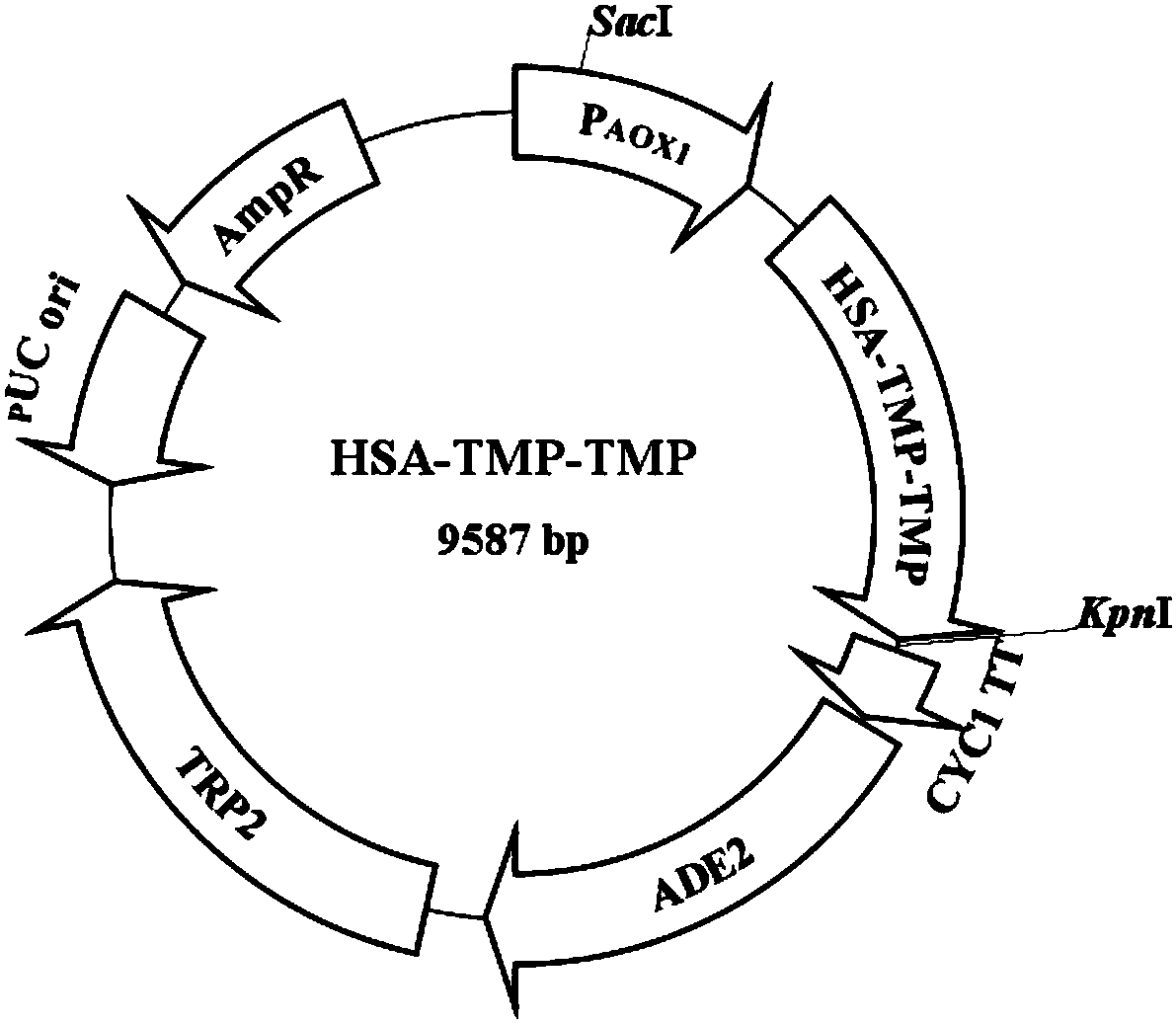
[0062] Example 1 Construction and expression of HSA-TMP fusion protein yeast expression vector
[0063] 1. Acquisition of p29-simple-TMP sequence
[0064] 1. First encode according to the preferred codon pair of Pichia pastoris (TMP) 2 optimized gene sequence.
[0065] 2. Entrust Dalian Takara Company to synthesize and optimize (TMP) 2 gene, (TMP) 2 The DNA sequence of the DNA sequence is shown in SEQ ID NO: 1, and it is loaded into p29-simple (p29-simple plasmid vector provided by Dalian Takara Company), and the vector p29-simple-(TMP) is obtained 2 .
[0066] 3. Wherein p29-simple-TMP already contains L, that is, the connecting peptide, the LDNA sequence is GGCGGCGGCGGTTCCGGACTGGAGCCCAAGAGCTGCGACAAGACCCACACCTGCCCTCCCTGCGAATTCGGTGGTGGCGG CAGC, and the amino acid sequence is GGGGSGLEPKSCDKTHTCPPCEF GGGGS.
[0067] 2. Cloning of fragments of HSA cDNA:
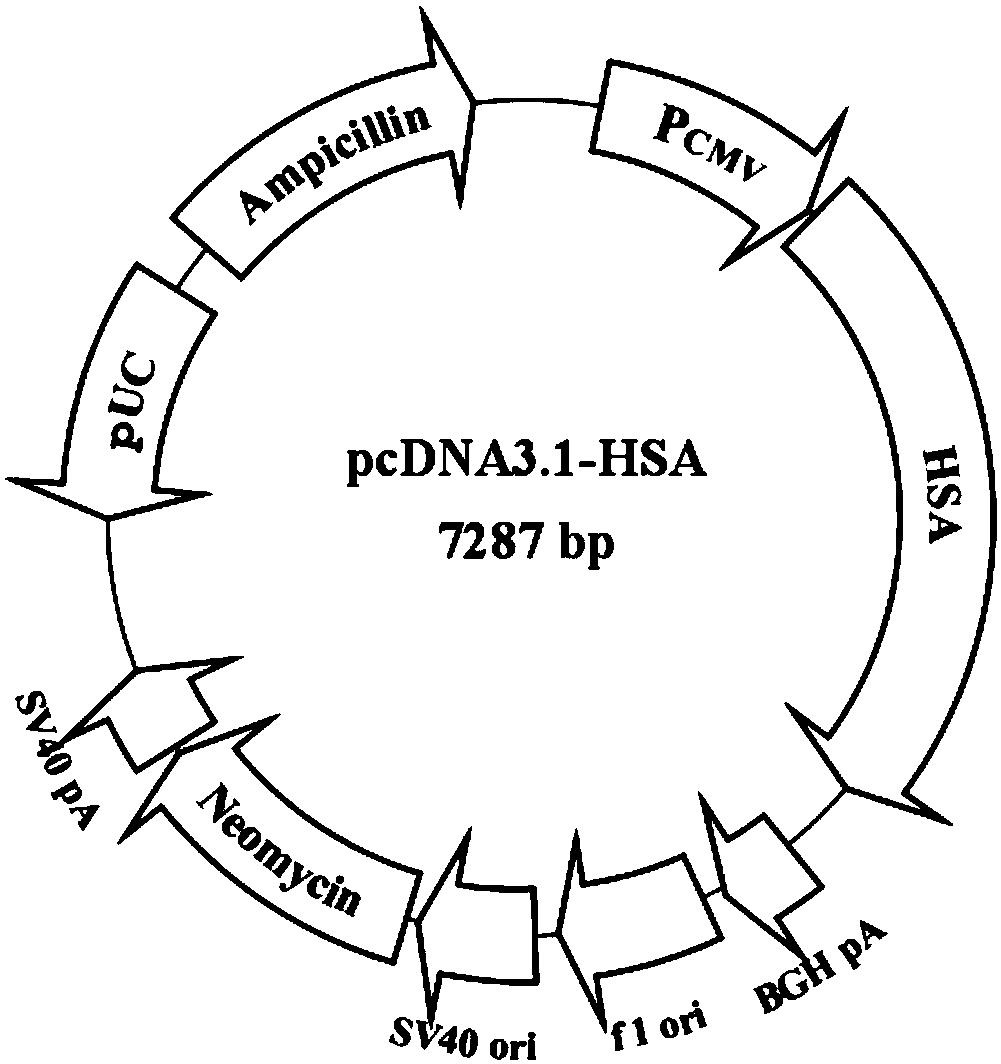
[0068] Obtained from plasmid pcDNA3.1-HSA clone (HSA complete gene sequence was synthesized by Bao Biological Company, p...
Embodiment 2
[0081] Example 2 HSA-(TMP) 2 Bioactivity detection of fusion protein
[0082] 1. Experimental process
[0083] Cell plating, c-mpl, c-fos, pAdVAntage, Renilla, four plasmid co-transfection. 48 hours after transfection, the positive control, blank control and test samples were added to detect firefly luciferase activity and Renilla luciferase activity, respectively, and calculate the fluorescence ratio.
[0084] Positive control: TPIAO TPIAO (recombinant human thrombopoietin injection) purchased from Shenyang Sansheng Pharmaceutical Co., Ltd.
[0085] Blank control: serum
[0086] Fluorescence ratio = firefly fluorescence value / renilla fluorescence value.
[0087] For specific steps, see Chinese patent (CN201310084212).
[0088] 2. Experimental results
[0089] Table 1 HSA-(TMP) 2 Bioactivity test results of fusion protein
[0090] test group
relative fluorescence ratio
Blank control group
1.00±0.02
positive control group
1.44±0.12 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com