Minitype optical derivatization device for aflatoxin and sulfonamides
A technology of sulfonamides and aflatoxins, which is applied in the field of optical detection, can solve the problems of reduced light derivation efficiency, reduced band broadening, and short light source life, and achieves the goal of reducing the volume of the derivation pool, shortening the analysis time, and reducing production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
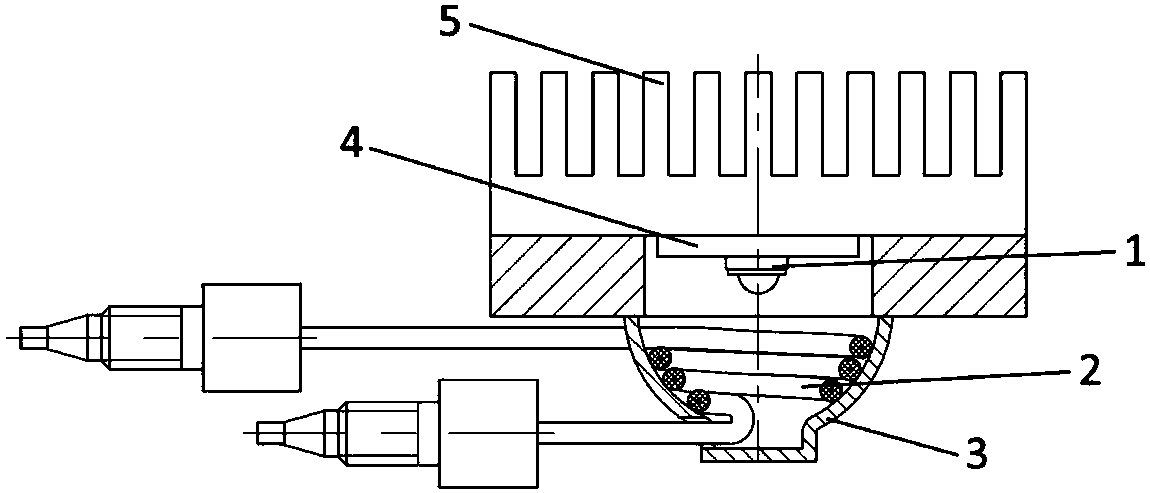
[0035] A post-column light derivatizer for high performance liquid chromatography (HPLC), the excitation light source is a high-power central wavelength 365nm ultraviolet LED, the bandwidth (FWHM) is 10nm, the divergence angle is 60°, and the rated current is 800mA; the derivation reaction tube is highly transparent The FEP tube has an inner diameter of 0.25 mm, an outer diameter of 1 / 16 inch, and a length of 18 cm. The volume of the derivation pool is 10 μL, and the distance between the derivation reaction tube and the LED light source is 7 mm; the derivation reaction tube is spirally wound on the inner surface of the reflective bowl and fixed with a thin steel wire; The LED substrate and heat sink are made of metal aluminum. The photochemical derivatizer was connected with Shimadzu RF-20A fluorescence detector to evaluate its performance. The liquid chromatography conditions are: chromatographic column: C18 column, 4.6mm×150mm×5μm; mobile phase A methanol: mobile phase B pur...
Embodiment 2
[0040] The photochemical derivatizer as described in Example 1 was combined with Shimadzu RF-20A fluorescence detector to analyze and detect 4 kinds of sulfa drugs. RF-20A has an excitation wavelength of 230nm and an emission wavelength of 400nm.
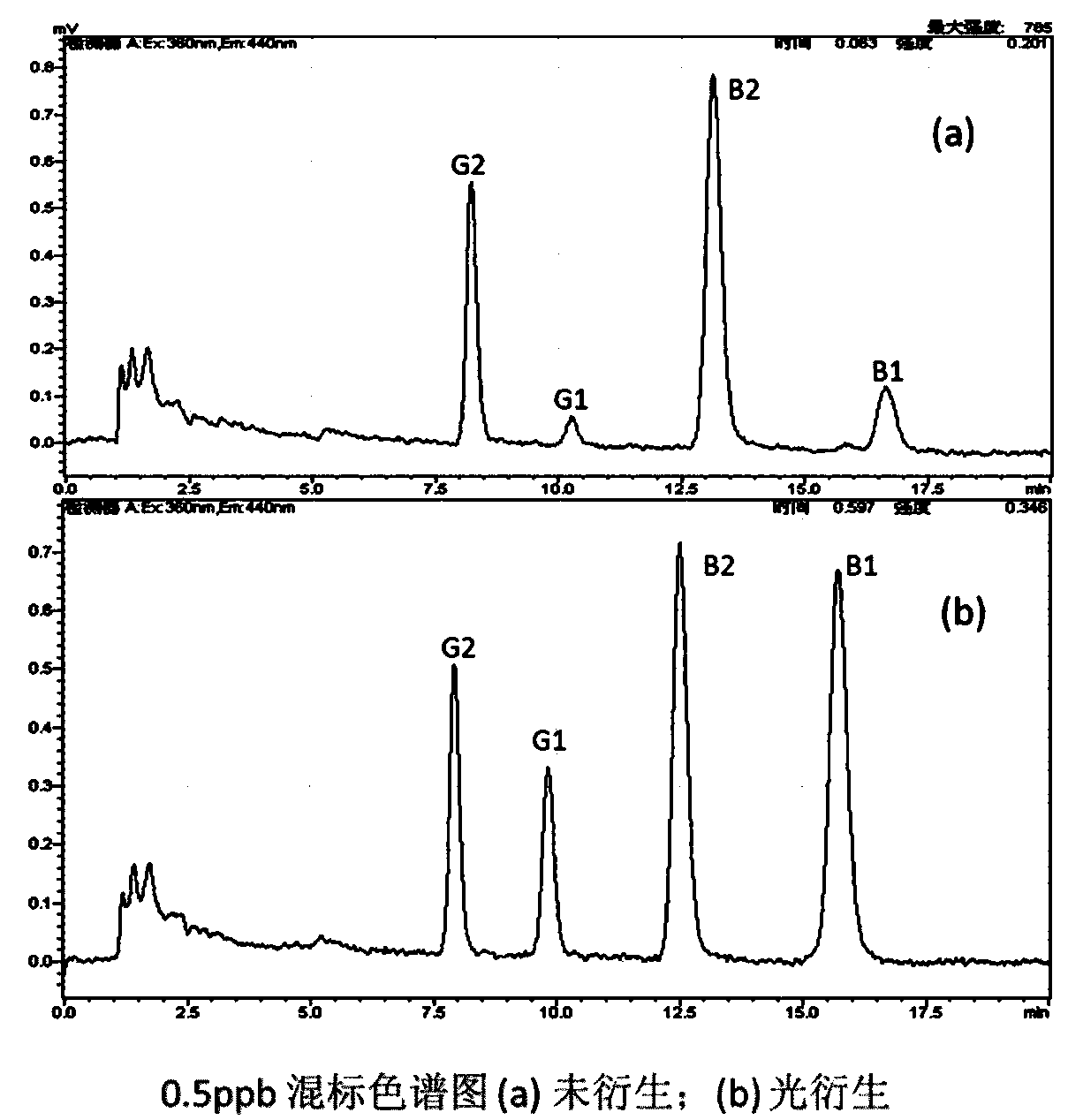
[0041] Experimental results: 5ppb sulfadiazine (SDZ) was injected, and RF-20A was not detected when the photochemical derivatizer was not connected; after the photochemical derivatizer was connected, 5ppb sulfadiazine (SDZ) could be detected, and the detection signal-to-noise ratio was 15.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Center wavelength | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com