Primer, detection kit and detection method for screening SCID genetic diseases
A genetic disease and kit technology, applied in the field of newborn genetic disease genetic screening, can solve problems such as the lack of newborn SCID screening technology system and the lack of clinical symptoms of SCID patients, so as to achieve stable blood card samples and avoid false positives. Positive result, easy storage effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Specific primers and gene detection kits for SCID genetic disease screening
[0035] 1. Design and synthesis of primers and probes:
[0036] According to the UCSCHumanGeneSorter on the UCSC website to query TRECs and internal reference Cα gene sequences (http: / / genome.ucsc.edu / cgi-bin / hgNear), use Primer3.0 to design fluorescent quantitative PCR upstream and downstream primers and probes on TRECs and Cα genes . The selected primers have good specificity for gene binding and high PCR amplification efficiency. The 5' end of the TRECs probe is a FAM fluorescent group, and the 3' end is a TAMRA fluorescent group; the 5' end of the Cα gene probe is a VIC fluorescent group, and the 3' end is a TAMRA fluorescent group. At the same time, the present invention also sets primers and probes for external control. Primer sequences are shown below.
[0037] TRECs target amplification upstream primer SEQ ID NO.1:
[0038] 5`-gtttttgtaaaggtgcccactcc-3`
[0039] TRECs t...
Embodiment 2
[0070] Embodiment 2: the use of kit in embodiment 1
[0071] 1. Extraction of sample genomic DNA
[0072] (1) Use a 3.0mm hole punch to punch 3 blood cards from the blood card and put them into a 1.5ml EP tube.
[0073] (2) Add 1000 μl ddH2O to the above-mentioned EP tube containing the blood card, vortex and oscillate, let it stand for 15 minutes, centrifuge at 12000 rpm for 2 minutes, discard the supernatant, if the color of the blood film is still very dark, wash it again once.
[0074] (3) Add 200 μl of 5% chelex100 (sigma) solution to the above-mentioned EP tube, shake and centrifuge and incubate at 56°C for 30 minutes, take it out and shake it for 1 minute, then centrifuge it, put it at 100°C for 8 minutes, take it out and shake it vigorously, and centrifuge it at 12000rpm for 2 minutes, take it out Store at 4°C for later use.
[0075] 2. PCR amplification
[0076] Using the two sets of PCR reaction solutions in Example 1, the template DNA obtained in the step 1 was r...
Embodiment 3
[0080] Embodiment 3: sample detection
[0081] 1. Select 4 cases of infant dried blood films with known clinical diagnosis results, and extract sample DNA. The operation is as follows:
[0082] (1) Use a 3.0mm hole punch to punch 3 blood cards from each of the 4 cases of dried blood and put them into 4 corresponding 1.5ml EP tubes.
[0083] (2) Add 1000 μl ddH2O to the above EP tube containing the blood card, vortex and oscillate, let stand for 15 minutes, centrifuge at 12000 rpm for 2 minutes, discard the supernatant, if the color of the blood card is still very dark, repeat washing once.
[0084] (3) Add 200 μl of 5% chelex100 (sigma) solution to each of the above EP tubes, shake and centrifuge and incubate at 56°C for 30 minutes, take it out and shake it for 1 minute, then centrifuge it, put it at 100°C for 8 minutes, take it out and shake it vigorously, and then centrifuge at 12000rpm for 2 minutes. Take it out and put it at 4°C for later use.
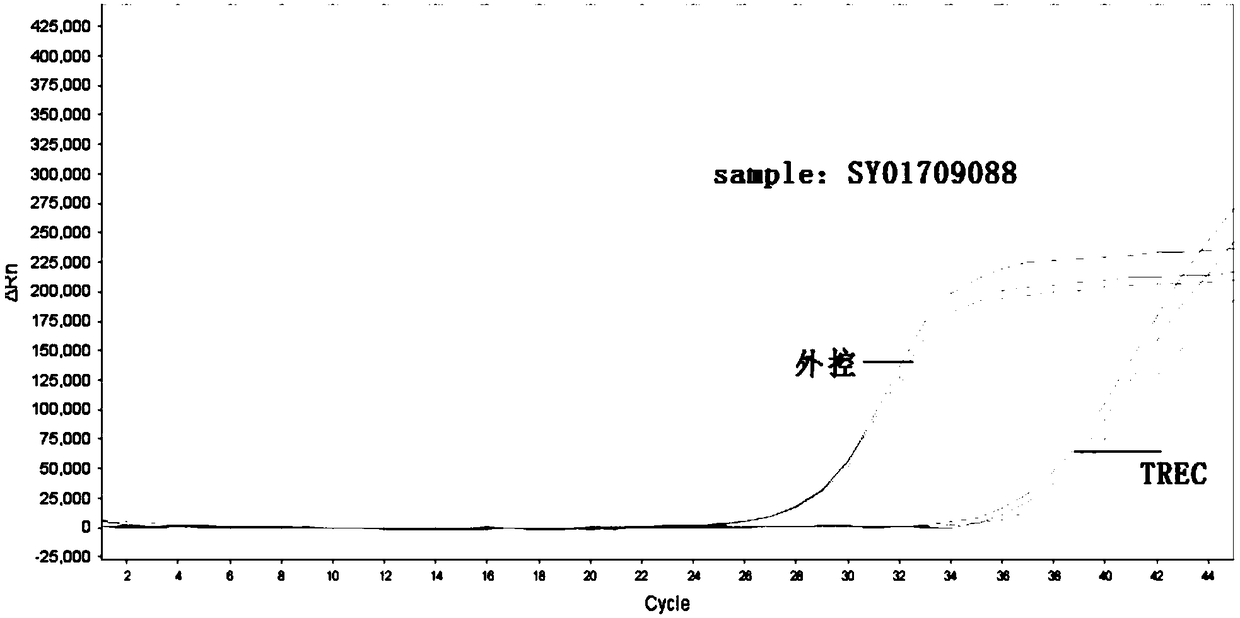
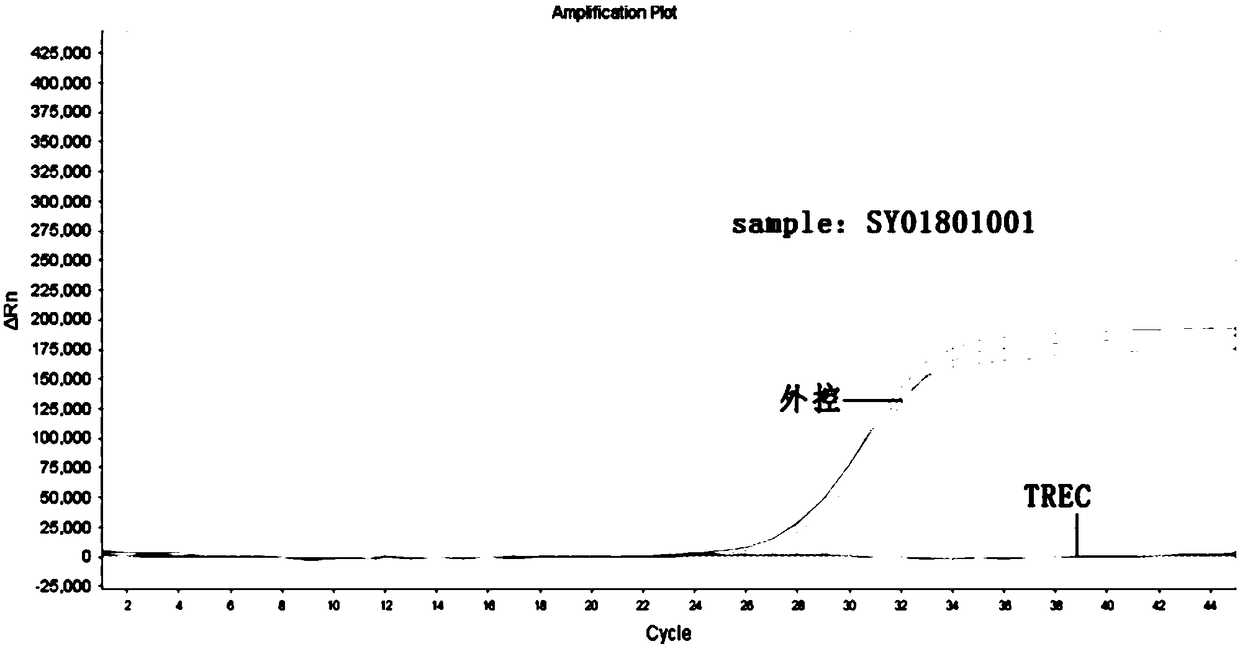
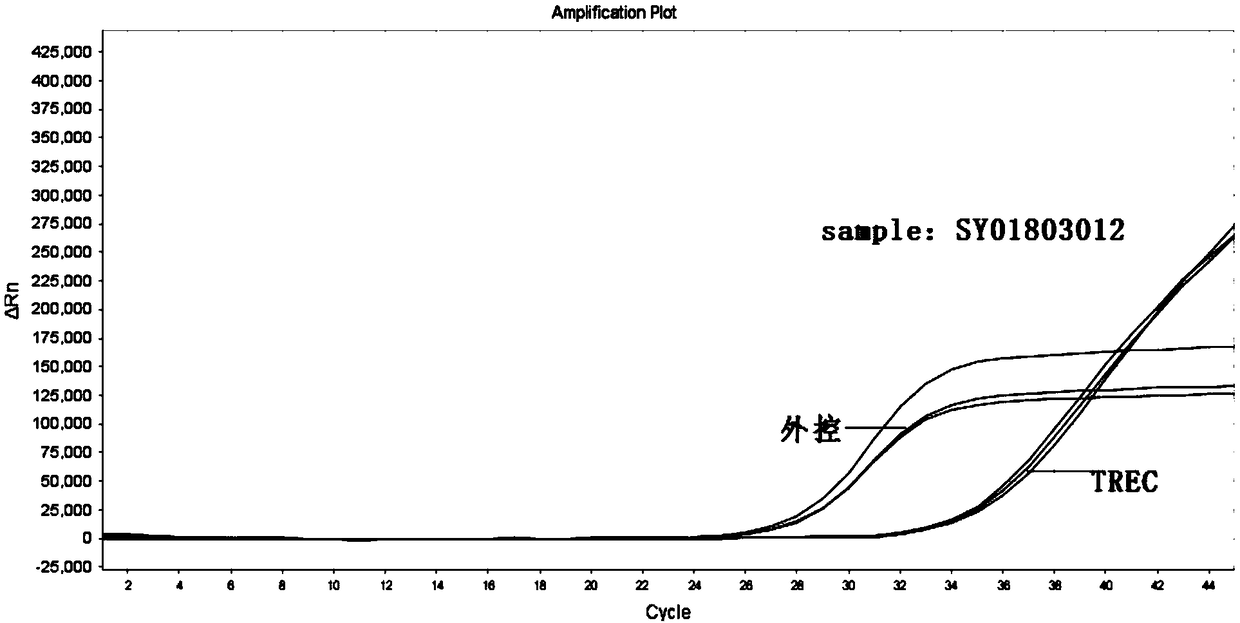
[0085] Table 1 Sample inf...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com