Synthesis method of 2H-azirines derivative
A synthetic method and allylidine technology, applied in the direction of steroids, organic chemistry, etc., can solve the problems of limited adaptability of reaction substrates, harsh conditions, complicated operation, etc., and achieve wide adaptability of substrates, mild reaction conditions, The effect of low process cost
Active Publication Date: 2019-03-08
NANCHANG HANGKONG UNIVERSITY
View PDF1 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Such a synthetic strategy has the disadvantages of multi-step reaction steps, complicated operation, harsh conditions, high cost, and limited adaptability of reaction substrates.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0031] The present invention will be further described in detail below in conjunction with specific embodiments.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
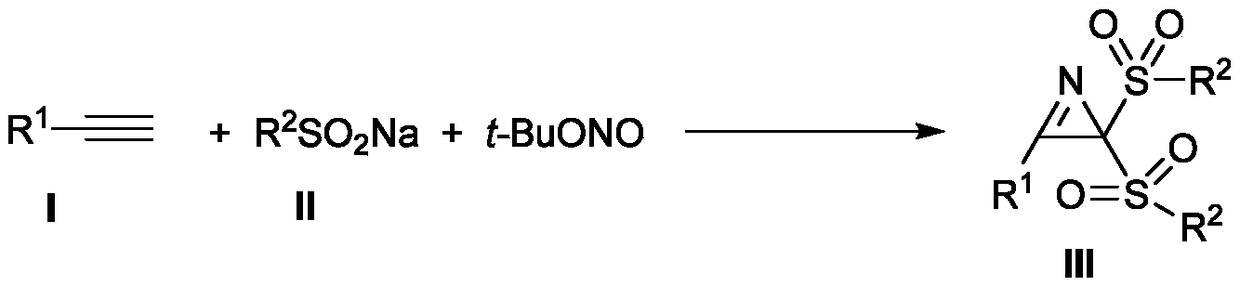
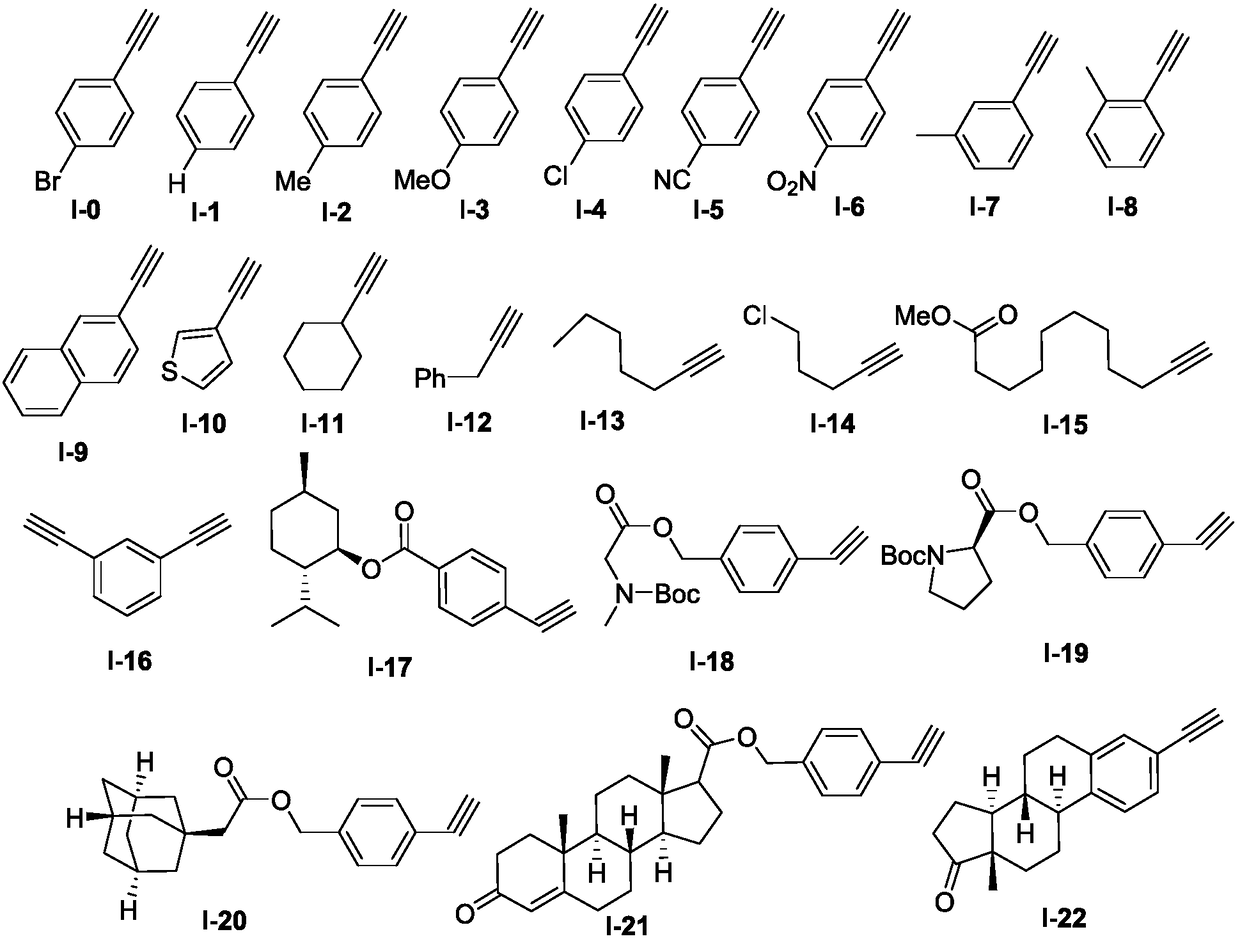
The invention discloses a novel method for synthesizing a 2H-azirines derivative. The method comprises the following steps: adding an alkyne compound shown as a formula I and sodium sulfonate shown asa formula II into a Schlenk sealing pipe reactor in sequence; then adding tert-butyl nitrite (t-BuONO) and an organic solvent; heating and stirring to react in an oil bath under a protection condition of an inert atmosphere; after finishing TLC or GC-MS monitoring reaction, carrying out post-treatment to obtain the 2H-azirines derivative shown as a formula III. The method has the advantages of easiness of obtaining raw material sources, simple technological route, moderate reaction conditions, low technological cost, wide substrate applicable range and high yield.
Description
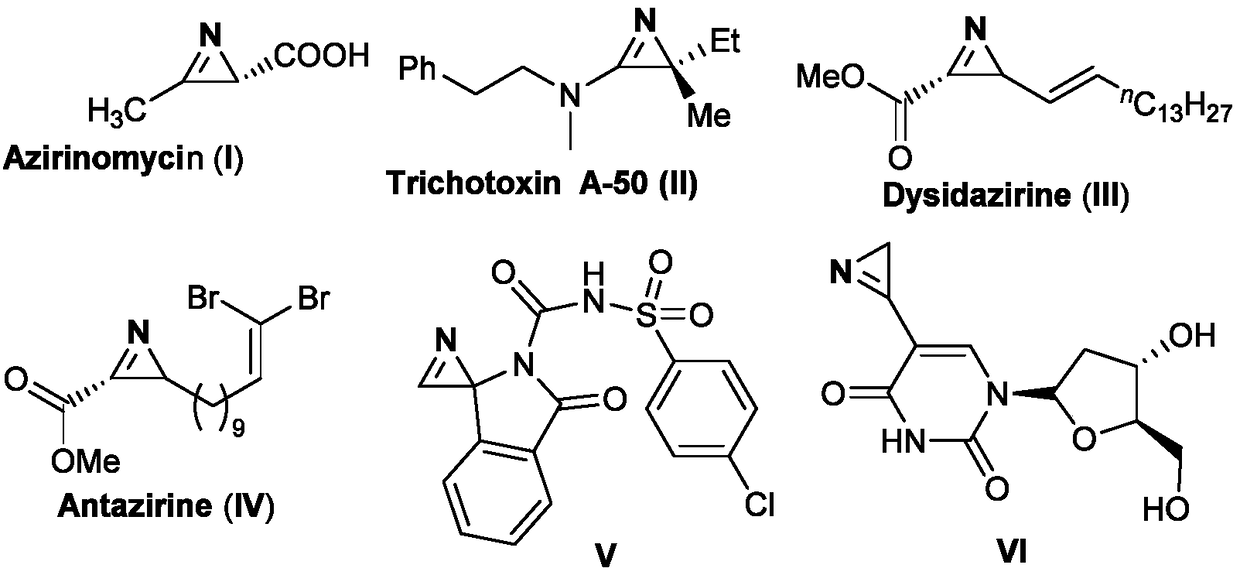
Technical field [0001] The invention belongs to the technical field of organic synthesis, and specifically relates to a synthesis method of 2H-aziridine derivatives. Background technique [0002] 2H-aziridine is the smallest unsaturated aza three-membered ring structure that exists in nature. They are widely present in natural products, medicines, agricultural chemicals, and organic materials. For example, the prior art has reported amphibian Azirinomycin (Azirinomycin) and other compounds having the structures shown in the following formulae I-VI. [0003] [0004] Due to the widespread existence of 2H-aziridine derivatives in nature and their great potential in the construction of amino derivatives and nitrogen-containing heterocycles, chemists have conducted in-depth studies on such molecules. 2H-aziridine derivatives have been widely used in the synthesis of complex nitrogen-containing acyclic molecules and various nitrogen heterocyclic compounds, such as pyrroles, indoles, py...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D203/04C07D409/04C07J43/00
CPCC07D203/04C07D403/04C07D409/04C07J43/003
Inventor 胡明何兴一吴双李金恒
Owner NANCHANG HANGKONG UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com