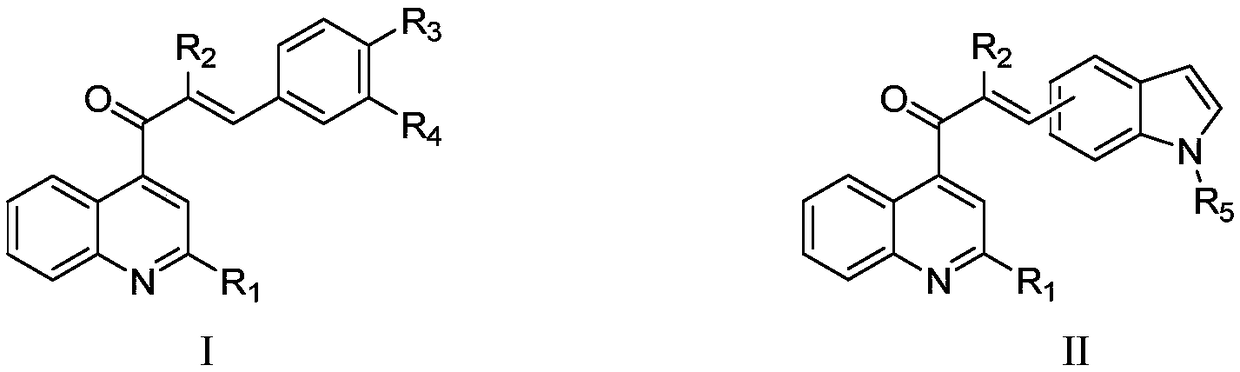
Quinoline substituted chalcone compound as well as preparation method and application thereof
A technology of chalcones and compounds, which is applied in the field of medicinal chemistry, can solve the problems of difficult synthesis, poor water solubility, and large toxic and side effects, and achieve the effects of low toxicity, stable metabolic properties, and excellent anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
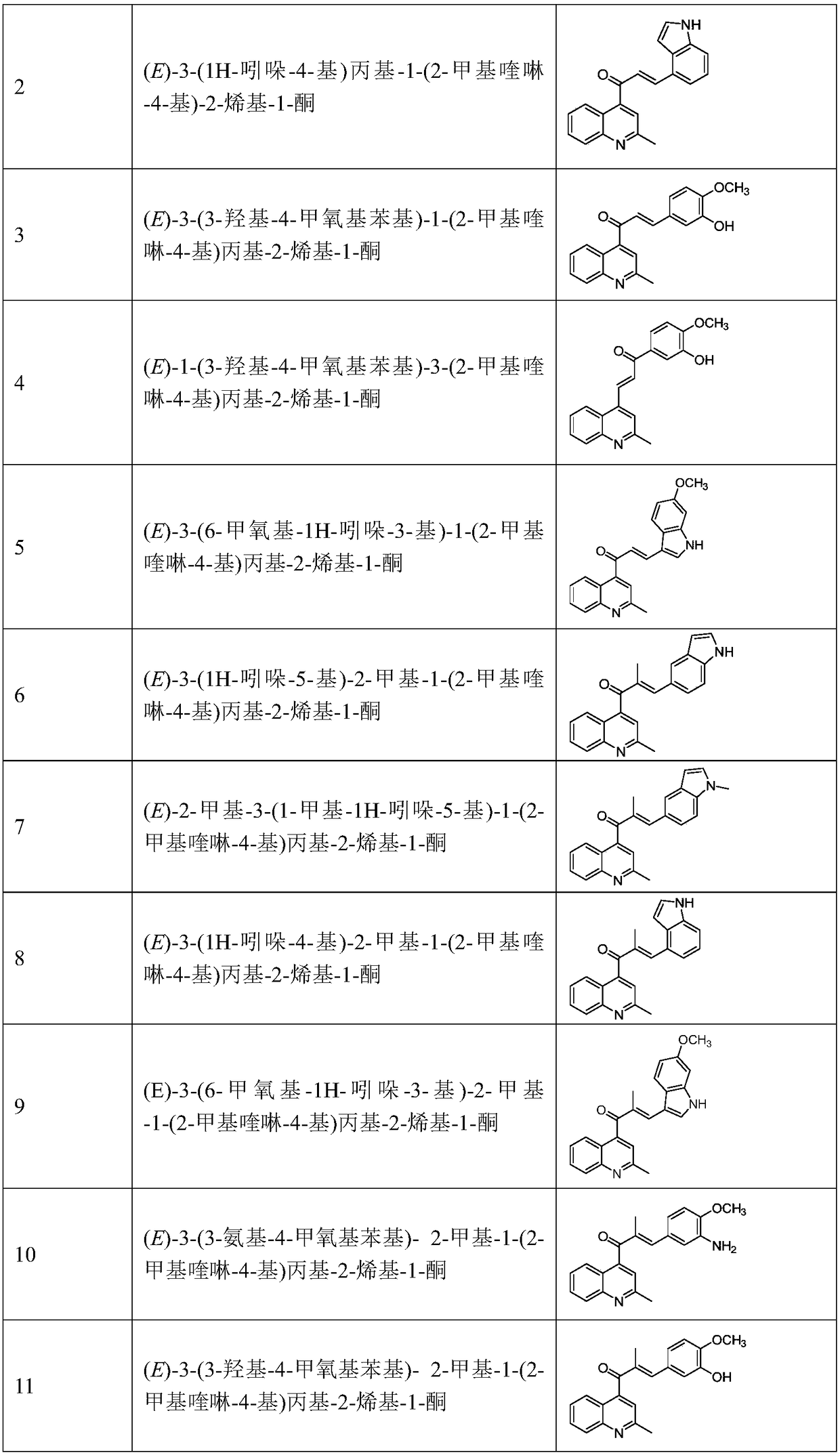
[0040] (E)-3-(1H-indol-5-yl)propyl-1-(2-methylquinolin-4-yl)-2-enyl-1-one
[0041] (a) Mix indole-2,3-dione (10g, 68mmol) and 85% KOH aqueous solution at 50°C and stir for 1h, then add 50mL of acetone dropwise, continue stirring for 15h, spin off the acetone, and adjust the pH to 3. Suction filtration and drying to obtain 11 g of 2-methylquinoline-4-carboxylic acid (white solid, 86.7%); 2-methylquinoline-4-carboxylic acid (500 mg, 2.7 mml) was dissolved in dichloromethane, Add dimethylhydroxylamine hydrochloride (310mg, 3.2mmol), triethylamine (440μL, 3.2mmol), EDCI (1g, 5.4mmol), catalytic amount of DMAP, stir at room temperature for 2h, dilute with water, extract with dichloromethane (50mL ×3), the organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated to obtain 510 mg of N-methoxy-N,2-dimethylquinoline-4-carboxamide, with a yield of 83.1%; (b ) Dissolve N-methoxy-N,2-dimethylquinoline-4-carboxamide (500m...
Embodiment 2
[0044]
[0045] (E)-3-(1H-indol-4-yl)propyl-1-(2-methylquinolin-4-yl)-2-enyl-1-one
[0046] According to the operation of Example 1 (c), 63 mg of yellow solid was obtained, and the yield was 50%; 1 H NMR (300MHz, CDCl 3 )δ9.11(s,1H),8.11(dd,J=8.2,4.6Hz,2H),7.97(d,J=16.1Hz,1H),7.78-7.66(m,1H),7.53(d,J =7.1Hz,1H),7.48(d,J=3.4Hz,1H),7.44(m,2H),7.38(s,1H),7.35-7.27(m,1H),7.19(t,J=7.8Hz ,1H),6.74(s,1H),2.81(s,3H); 13 C NMR (75MHz, CDCl 3 )δ194.85,158.04,147.94,146.93,145.16,135.97,129.53,128.51, 126.78,126.33,125.77,125.64,124.84,122.58,121.44,119.69,114.10,100.73, 76.99,76.56,76.14,24.83;ESI-MS m / z:312.1calcd for C 21 h 16 N 2 O[M+H] + 313.1.
Embodiment 3
[0048]
[0049] (E)-3-(3-Hydroxy-4-methoxyphenyl)-1-(2-methylquinolin-4-yl)propyl-2-enyl-1-one
[0050] According to the operation of Example 1(c), 120 mg of a yellow solid was obtained with a yield of 70.5%; 1 H NMR (300 MHz, DMSO) δ9.26 (s, 1H), 8.03 (d, J = 9.0Hz, 2H), 7.76 (t, J = 7.6Hz, 1H), 7.65 (s, 1H), 7.57 ( t,J=7.9Hz,1H),7.50(d,J=16.0Hz,1H),7.33–7.22(m,2H),7.20 (d,J=6.3Hz,1H),6.97(d,J=8.4 Hz,1H),3.83(s,3H),2.74(s,3H); 13 C NMR(75 MHz,DMSO)δ193.80,158.59,150.87,147.82,147.55,146.77,144.27,129.66, 128.80,126.94,126.61,124.96,123.60,122.60,122.37,120.42,114.68,111.91, 55.65,40.34,40.06, 39.79, 39.51, 39.23, 38.95, 38.68, 24.81; ESI-MS m / z: 319.1 calcd for C 21 h 17 NO 3 [M+H] + 320.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com