Compound licorice tablet and preparation method thereof
A technology of compound licorice tablets and licorice extract powder, which is applied in the direction of medical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as difficult industrialization, complicated operation, and difficult industrialization , to increase the effect of adsorption and fixation, increase the content of volatile matter, and make it less volatile
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] (1) Crushing and sieving: Glycyrrhiza extract powder, poppy fruit extract powder, sodium benzoate, magnesium oxide, starch, calcium hydrogen phosphate, talcum powder, micronized silica gel are pulverized through a 30-mesh sieve with the raw and auxiliary materials;
[0058] (2) Weighing: take by weighing licorice extract powder 1125g by the amount of preparing 10000 compound licorice tablets, poppy fruit extract powder 40g, sodium benzoate 20g, magnesium oxide 40g, starch 300g, calcium hydrogen phosphate 300g, talcum powder 100g, Micropowder silica gel 5g, spare;
[0059] (3) Initial mixing: 1125g of licorice extract powder in step (2), 40g of poppy fruit extract powder, 20g of sodium benzoate, 40g of magnesium oxide, 300g of starch, 300g of calcium hydrogen phosphate, and 100g of talcum powder, mixed for 5 minutes;
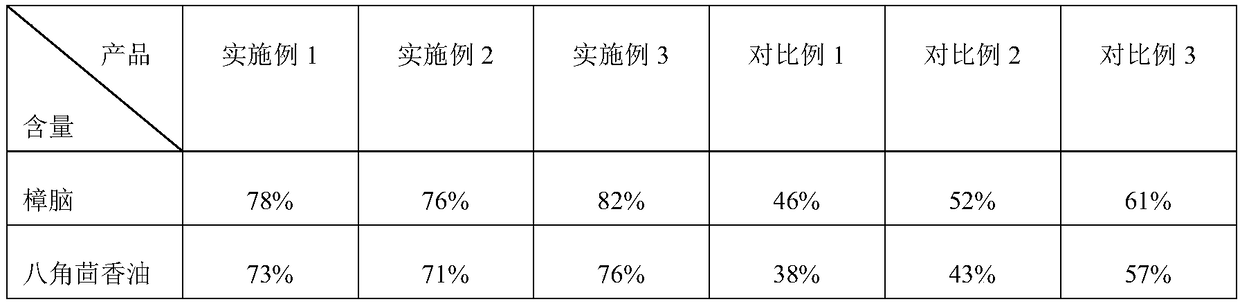
[0060] (4) Dissolving: Dissolve 20 g of camphor in 20 g of star anise oil, add 80 g of beta-cyclodextrin with an average particle size of about 1 μm under...
Embodiment 2
[0068] (1) Pulverize and sieve: Glycyrrhiza extract powder, poppy fruit extract powder, sodium benzoate, magnesium oxide, starch, talcum powder, micropowder silica gel are pulverized through a 40-mesh sieve with raw and auxiliary materials;
[0069] (2) Weighing: Take by weighing 1125g of licorice extract powder, 40g of poppy fruit extract powder, 20g of sodium benzoate, 120g of magnesium oxide, 300g of starch, 100g of talcum powder, 50g of micronized silica gel by preparing 10000 compound licorice tablets, and set aside ;
[0070] (3) Initial mixing: 1125g of licorice extract powder in step (2), 40g of poppy fruit extract powder, 20g of sodium benzoate, 120g of magnesium oxide, 300g of starch, and mix for 3min;
[0071] (4) Dissolving: Dissolve 20 g of camphor in 20 g of star anise oil, add 160 g of beta-cyclodextrin with an average particle size of 5 μm under stirring for adsorption, ultrasonication for 10 min, and leave in airtight for 2 h; add the mixture in step (3) and r...
Embodiment 3
[0079] (1) Pulverize and sieve: Glycyrrhiza extract powder, poppy fruit extract powder, sodium benzoate, magnesium oxide, starch, calcium hydrogen phosphate, talcum powder, micropowder silica gel are pulverized through a 40-mesh sieve with raw and auxiliary materials;
[0080] (2) Weighing: Take by weighing 1125g of licorice extract powder, 40g of poppy fruit extract powder, 20g of sodium benzoate, 120g of magnesium oxide, 300g of calcium hydrogen phosphate, 100g of talcum powder, and 50g of micronized silica gel by preparing 10,000 compound licorice tablets. ,spare;
[0081] (3) Initial mixing: 1125g of licorice extract powder in step (2), 40g of poppy fruit extract powder, 20g of sodium benzoate, 120g of magnesium oxide, 300g of calcium hydrogen phosphate, 50g of talcum powder, and mix for 10min;
[0082] (4) Dissolving: Dissolve 20 g of camphor in 20 g of star anise oil, add 120 g of beta-cyclodextrin with an average particle size of 2 μm under stirring for adsorption, stir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com