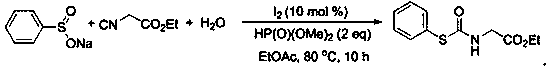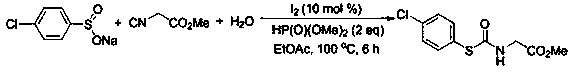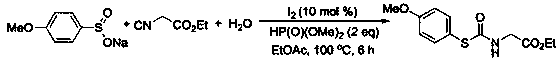Preparation method of thiocarbamate compound
A technology of thiocarbamate and carbamate, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as unfavorable actual production, complex and cumbersome operation, instability and easy oxidation, and achieve Avoid the use of metal reagents, process safety, and the effects of avoiding the use of toxic phosgene and carbon monoxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] At room temperature, sodium benzenesulfinate (0.2mmol), ethyl isocyanoacetate (0.4mmol), water (0.6mmol), acetonitrile (1.5mL), molecular iodine (0.02mmol), and dimethyl phosphite (0.4mmol), mix well, then in 100 o After heating and stirring under the condition of C for 6 hours, after the reaction was detected by TLC, 2 mL of water was added, and then extracted with ethyl acetate (4 mL) for 3 times, and the extract was concentrated under reduced pressure at 0.08 Mpa to solvent-free to obtain the crude product. Then wash with a mixed eluent of petroleum ether and ethyl acetate with a volume ratio of 5:1, and perform flash column chromatography on a silica gel column to obtain 83% thiocarbamate, 39.7 mg of a white solid.
[0030] 1 H NMR (500 MHz, CDCl 3 ): δ 7.61 – 7.59 (m, 2H), 7.47 – 7.42 (m, 3H),6.00 (s, 1H), 4.21 (q, J = 7.2 Hz, 2H), 4.04 (d, J = 5.1 Hz, 2H), 1.28 (t, J = 7.2 Hz, 3H); 13 C NMR (125 MHz, CDCl 3 ): δ 169.3, 166.9, 135.6, 130.0...
Embodiment 2
[0032]
[0033] At room temperature, sodium benzenesulfinate (0.2mmol), ethyl isocyanoacetate (0.4mmol), water (0.6mmol), ethyl acetate (1.5mL), molecular iodine (0.02mmol ), and diethyl phosphite (0.4mmol), mix well, then in 100 oAfter heating and stirring for 6 hours under the condition of C, after the reaction was detected by TLC, 2 mL of water was added, and then extracted 3 times with ethyl acetate (4 mL). Then wash with a mixed eluent of petroleum ether and ethyl acetate with a volume ratio of 5:1, and perform flash column chromatography on a silica gel column to obtain 75% thiocarbamate, 35.9 mg of a white solid.
Embodiment 3
[0035]
[0036] At room temperature, sodium benzenesulfinate (0.2mmol), ethyl isocyanoacetate (0.4mmol), water (0.6mmol), ethyl acetate (1.5mL), molecular iodine (0.02mmol ), and diisopropyl phosphite (0.4mmol), mix well, and then in 100 o After heating and stirring for 6 hours under the condition of C, after the reaction was detected by TLC, 2 mL of water was added, and then extracted 3 times with ethyl acetate (4 mL). Then wash with a mixed eluent of petroleum ether and ethyl acetate with a volume ratio of 5:1, and perform flash column chromatography on a silica gel column to obtain 60% thiocarbamate, 28.7 mg of a white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com