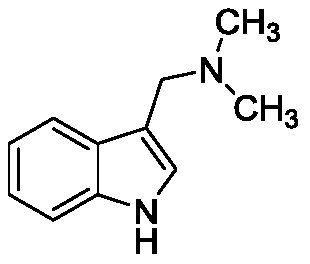
Phylloline derivatives and their preparation methods and uses
A technology of anophylline and its derivatives, which is applied in the field of anophylline derivatives and its preparation, can solve the problems of high production cost, environmental pollution, and low yield, and achieve the effect of good anti-plant virus and germ activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of the anophylline derivative shown in the chemical structural formula general formula I is as follows:
[0035] The prepared anophylline derivative shown in general formula I has the following chemical structural formula:
[0036]
[0037] In the general formula I of the above chemical structural formula, R 1 is methyl, ethyl, n-propyl, isopropyl, n-butyl, tert-butyl, phenyl or benzyl, R 2 phenyl, 2-fluorophenyl, 3-fluorophenyl, 3,4-difluorophenyl, 2,6-difluorophenyl, 2,4-difluorophenyl, 2,3-difluorobenzene Base, 4-fluorophenyl, 2-chlorophenyl, 3-chlorophenyl, 3,4-dichlorophenyl, 2,6-dichlorophenyl, 2,4-dichlorophenyl, 2,3 -Dichlorophenyl, 2,5-dichlorophenyl, 4-chlorophenyl, 5-chloro-2-fluorophenyl, 2-bromophenyl, 3-bromophenyl, 4-bromophenyl, 4 -Iodophenyl, 2-nitrophenyl, 3-nitrophenyl, 4-nitrophenyl, 2-methylphenyl, 3-methylphenyl, 4-methylphenyl, 2-tri Fluoromethylphenyl, 3-trifluoromethylphenyl, 4-trifluoromethylphenyl, 3,5-bis(trifluo...
Embodiment 2
[0042] The preparation method of (N-((5-bromo-1-methyl-1H-indol-3-yl)(phenyl)methyl)pyridin-2-amine) shown in chemical structural formula I-1 is as follows:
[0043] The chemical structural formula I-1 of N-((5-bromo-1-methyl-1H-indol-3-yl)(phenyl)methyl)pyridin-2-amine is
[0044]
[0045] The concrete steps of its preparation method are as follows:
[0046] In the first step, at 0°C, add 0.64g or 16mmol of NaH into 10mL of DMF, stir and let it stand still, then suck out 5mL of the solvent DMF, then add 10mL of DMF, stand still after stirring and suck out 5mL of the solvent, and then Add 30-40 mL of DMF, 1.94 g (10 mmol) of 5-bromoindole, and 1.65 g (11.9 mmol) of iodomethane. After the addition is complete, move the mixture to room temperature for TLC detection. After the reaction is completed, slowly add water dropwise in an ice bath. Water phase with 30mL CH 2 Cl 2 Extract three times, wash the organic phase with water and saturated brine successively, combine the or...
Embodiment 3
[0049] The preparation method of (N-((5-bromo-1-methyl-1H-indol-3-yl) (4-fluorophenyl) methyl) pyridin-2-amine shown in chemical structural formula I-2 is as follows :
[0050] The chemical structural formula I-2 of N-((5-bromo-1-methyl-1H-indol-3-yl)(4-fluorophenyl)methyl)pyridin-2-amine is
[0051]
[0052] The concrete steps of its preparation method are as follows:
[0053] The first step, with embodiment 2;
[0054] In the second step, in an oil bath at 80°C, 0.376g or 4mmol of 2-aminopyridine, 0.496g or 4mmol of 4-fluorobenzaldehyde and 0.840g or 4mmol of the above-mentioned first step were added to the reactor under magnetic stirring conditions. After the addition of N-methyl-5-bromoindole, the system was controlled at 90°C and reacted for 3 hours. After the reaction was completed, 5mL of distilled water was added to the system for washing, and the washing was repeated three times. Chloromethane was extracted three times, the organic phases were combined, dried by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com