Preparation process of human immunoglobulin for intravenous injection
A human immunoglobulin and preparation process technology, applied in the field of biopharmaceuticals, can solve the problems of affecting the biological activity of intravenously injected human immunoglobulin, affecting the safety of clinical use of the product, and the inability to effectively remove viruses, so as to improve the appearance of the product, improve the Product safety, loss reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
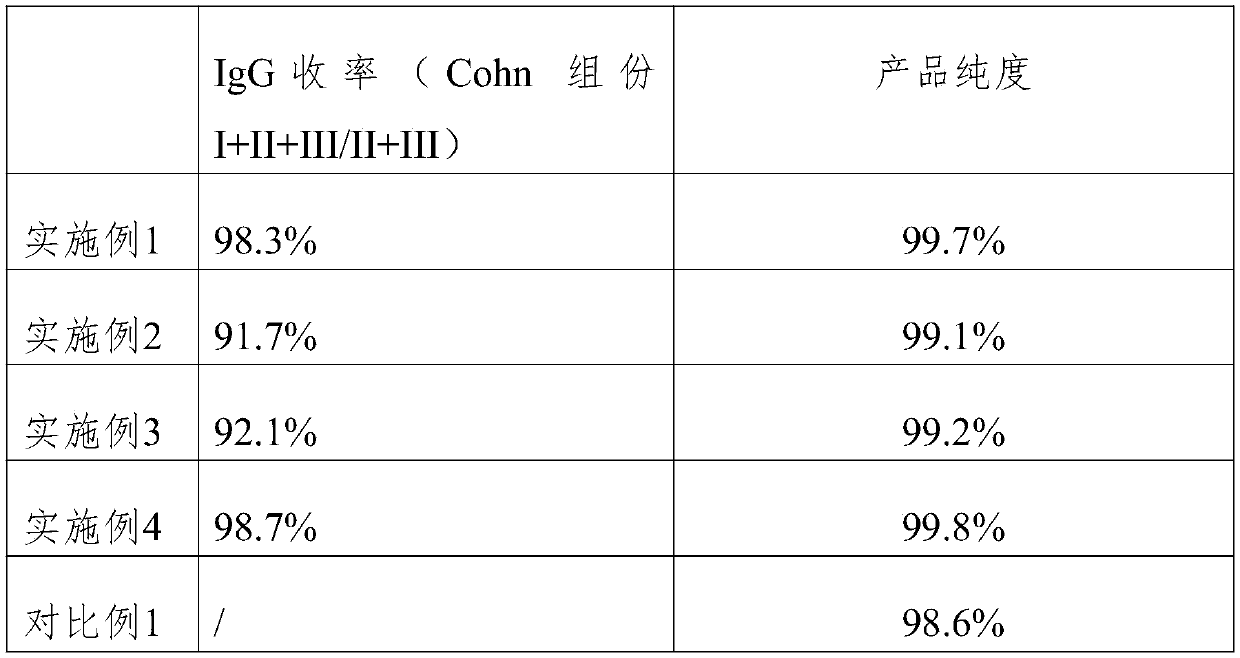
Embodiment 1
[0033] (1) Take 1kgCohn component I+II+III / II+III as raw material;
[0034] (2) Dissolve Cohn component I+II+III / II+III with 10 times water for injection, adjust pH=4.0 with acetic acid, and press filter to get the filtrate;
[0035] (3) in filtrate, add octanoic acid to make filtrate concentration be 20mmol / L, regulate filtrate pH to 4.8 with NaOH, add filter aid, press filtration;
[0036] (4) Adjust the pH of the filtered filtrate to 4.0, and incubate at 37° C. for 9 hours;
[0037] (5) The filtrate after hatching is first carried out EMD DEAE chromatography, then carries out EMD TMAE chromatography, controls twice chromatography speed 150cm / h, during DEAE chromatography, solution is adjusted the pH of 5.1, and during TMAE chromatography, solution Adjust to a pH of 5.9, and maintain a conductivity of 1.8mS / cm during two chromatographic runs;
[0038] (6) collect the flow-through liquid through two chromatography, carry out ultrafiltration dialysis concentration;
[0039]...
Embodiment 2
[0043] (1) Take 1kgCohn component I+II+III / II+III as raw material;
[0044] (2) Dissolve Cohn component I+II+III / II+III with 8 times water for injection, adjust pH=3.8 with acetic acid, and take the filtrate by deep filtration;
[0045] (3) in filtrate, add octanoic acid to make filtrate concentration be 20mmol / L, regulate filtrate pH to 4.8 with NaOH, add filter aid, press filtration;
[0046] (4) Adjust the pH of the filtered filtrate to 4.0, and incubate at 37° C. for 6 hours;
[0047] (5) The filtrate after hatching is first carried out EMD DEAE chromatography, then carries out EMD TMAE chromatography, controls twice chromatography speed 100cm / h, during DEAE chromatography, solution is adjusted to the pH of 4.8, and during TMAE chromatography, solution Adjust to a pH of 5.6, and maintain a conductivity of 1.6mS / cm during two chromatography;
[0048] (6) collect the flow-through liquid through two chromatography, carry out ultrafiltration dialysis concentration;
[0049]...
Embodiment 3
[0053] (1) Take 1kgCohn component I+II+III / II+III as raw material;
[0054] (2) Dissolve Cohn component I+II+III / II+III with 12 times water for injection, adjust pH=4.2 with acetic acid, and take the filtrate by deep filtration;
[0055] (3) in filtrate, add octanoic acid to make filtrate concentration be 20mmol / L, regulate filtrate pH to 4.8 with NaOH, add filter aid, press filtration;
[0056] (4) Adjust the pH of the filtered filtrate to 4.0, and incubate at 37° C. for 12 hours;
[0057] (5) The filtrate after hatching is first carried out EMD DEAE chromatography, then carries out EMD TMAE chromatography, controls twice chromatography speed 200cm / h, during DEAE chromatography, the pH of solution adjustment 5.4, during TMAE chromatography, solution Adjust to a pH of 6.2, and maintain a conductivity of 2.0 mS / cm during two chromatography;
[0058] (6) collect the flow-through liquid through two chromatography, carry out ultrafiltration dialysis concentration;
[0059] (7) ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com