Application of polygonum sinense polysaccharide to preparation of drugs for preventing and treating viral pneumonia
A technology of viral pneumonia and fotanmu, which is applied in the direction of antiviral agents, drug combinations, and pharmaceutical formulas, can solve the problems of drug efficacy and drug research on acute pneumonia of fotanmu polysaccharides that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Take 100g of Polygonum chinense Linn., pulverize it, and extract it by cold soaking with 95% ethanol for 3 times, put the dregs in a ventilated place at room temperature to dry, then extract it with hot water for 3 times, filter, combine the extracts, and concentrate , centrifuged, the supernatant was removed with trichloroacetic acid to remove free protein, centrifuged, the supernatant was dialyzed with water for 3 days, the dialysate was concentrated to a small volume, added ethanol until the alcohol content was 80%, centrifuged, precipitated and freeze-dried to obtain the total polysaccharide, the product was collected rate of more than 2.2%. The sugar content of the total polysaccharides of Fotan mother measured by the sulfuric acid-phenol method exceeds 60%; the uronic acid content of the total polysaccharides of Fo Tan mother measured by the m-hydroxybiphenyl method exceeds 20%; The protein content is not more than 10%, and the results are shown in Table 1.
[00...
Embodiment 2
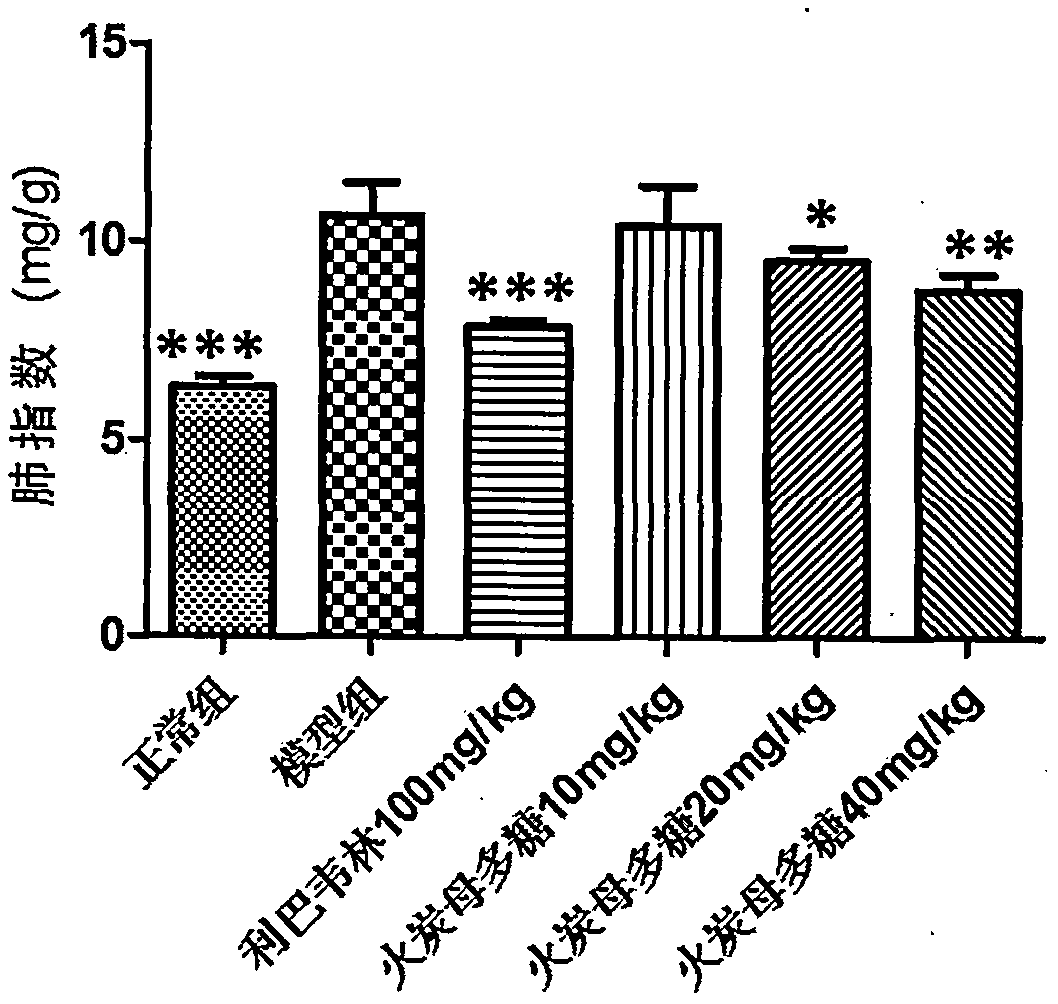
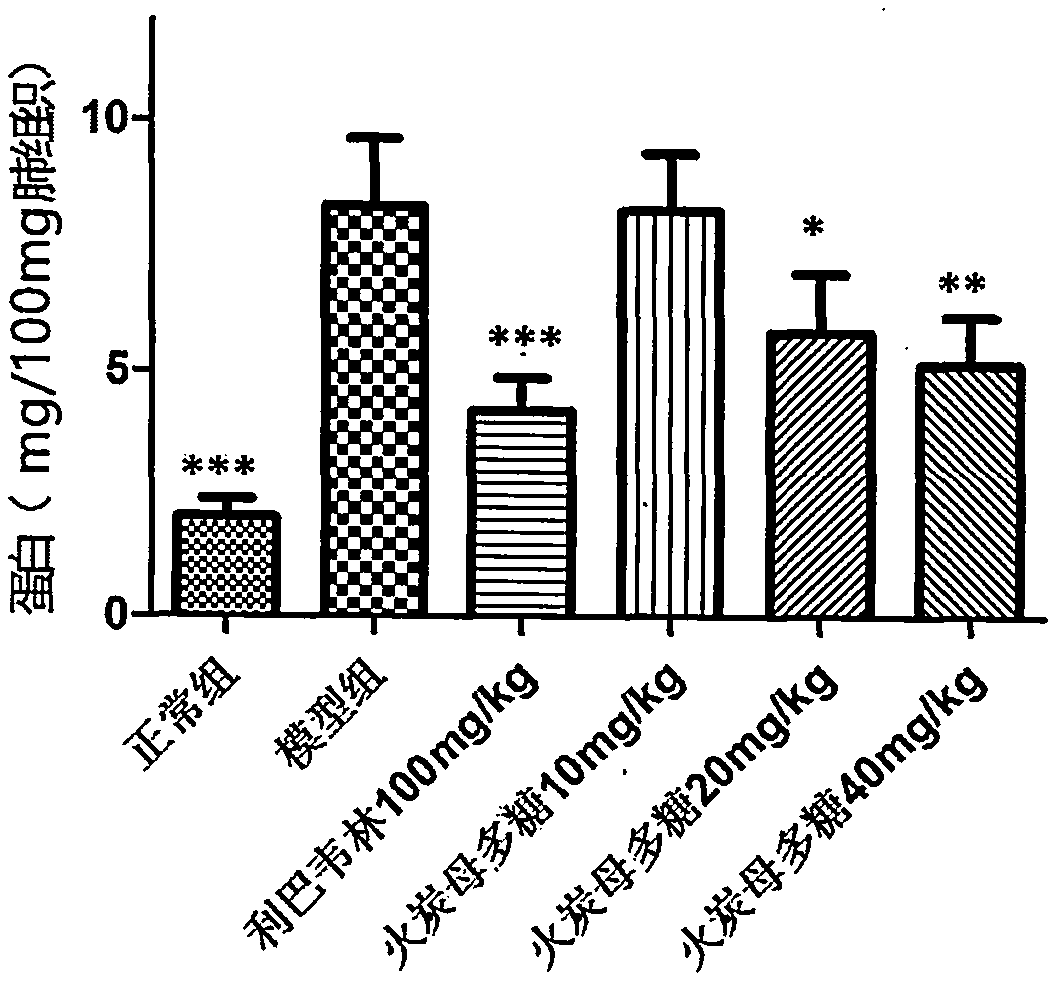
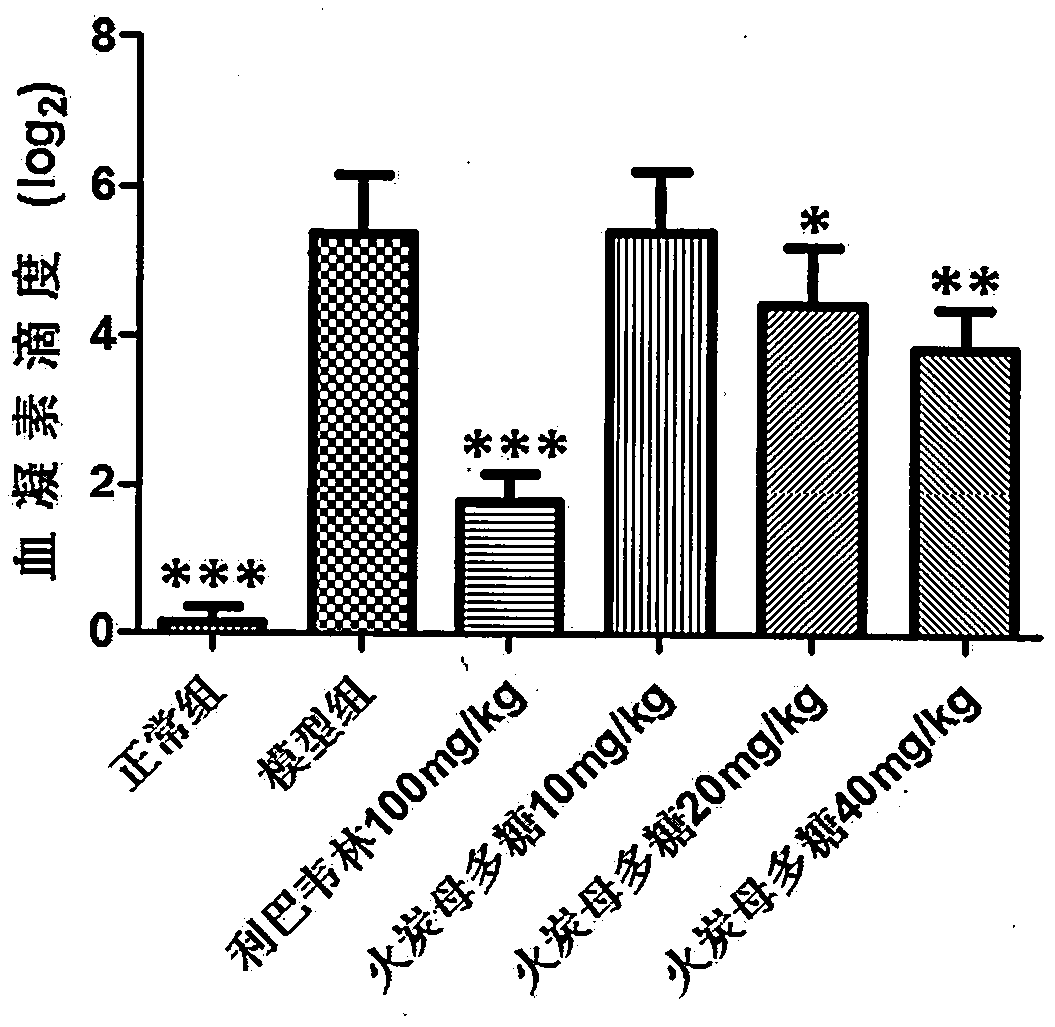
[0043] Thirty BALB / C mice (14-16g) were randomly divided into 6 groups (N, M, P, A, B, C) according to body weight: the N group was the normal group, the M group was the H1N1 virus model group, and the P group was the H1N1 virus model group. Positive drug ribavirin 100mg / kg, Group A received Fotanmu polysaccharide 10mg / kg, Group B received Fotanmu polysaccharide 20mg / kg, Group C received Fotanmu polysaccharide 40mg / kg, 6 rats in each group. All animals were anesthetized by propofol tail vein injection, and 30ul of 1640 culture solution was used as a control in group N; the other groups were infected with 5LD by nasal drip 50H1N1 virus liquid 30ul, intragastric administration two hours after H1N1 infection, N group and M group were given 0.5% CMC intragastric administration, as normal and virus control. Dosing once a day for four consecutive days. After 96 hours of H1N1 virus attack, the body weight was weighed, and the eyeballs were removed to collect blood. Carefully cut of...
Embodiment 3
[0050] 40 BALB / C mice (14-16g) were randomly divided into 4 groups (N, M, P, A) according to body weight: N group was the normal control group, M group was the H1N1 virus model group, and A group was Fotan mother polysaccharide group (PCP40mg / kg), the P group is ribavirin RIBAVIRIN100mg / kg, 10 in each group. All animals in the group are anesthetized by propofol, and the N group nasal drop 1640 medium 30ul is used as a control; nasal drop infection 10LD 50 H1N1 virus 30ul, A and P groups were given intragastric administration 2 hours after H1N1 infection, and N group and M group were given 0.5% CMC intragastric administration at the same time, as normal and virus control, administered once a day, continuously administered for seven days, and stopped. After the drug was observed for 14 days, the number of survival and death of the animals was recorded every day, and the life protection rate of the drug on mice with severe influenza infection was calculated;
[0051] The results ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com