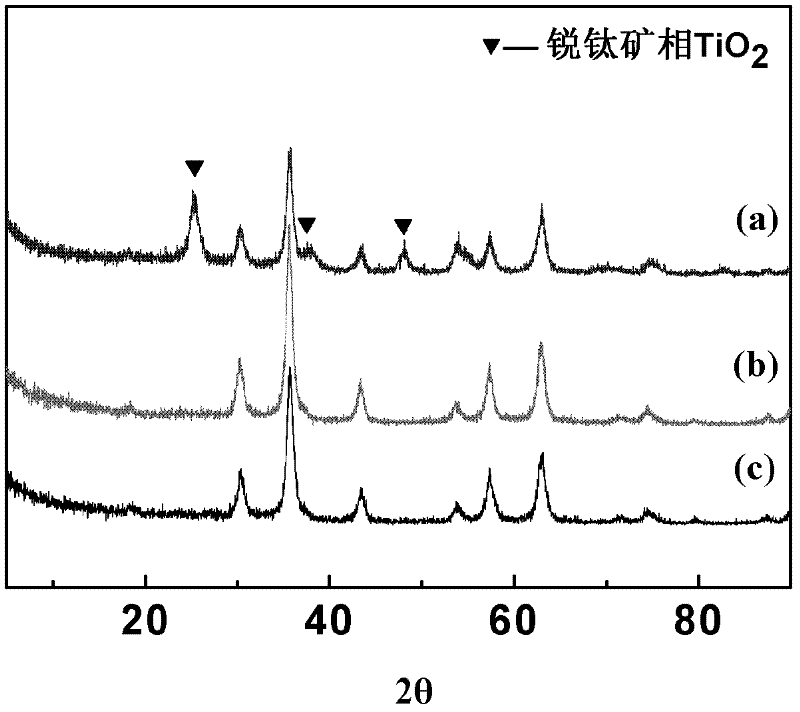
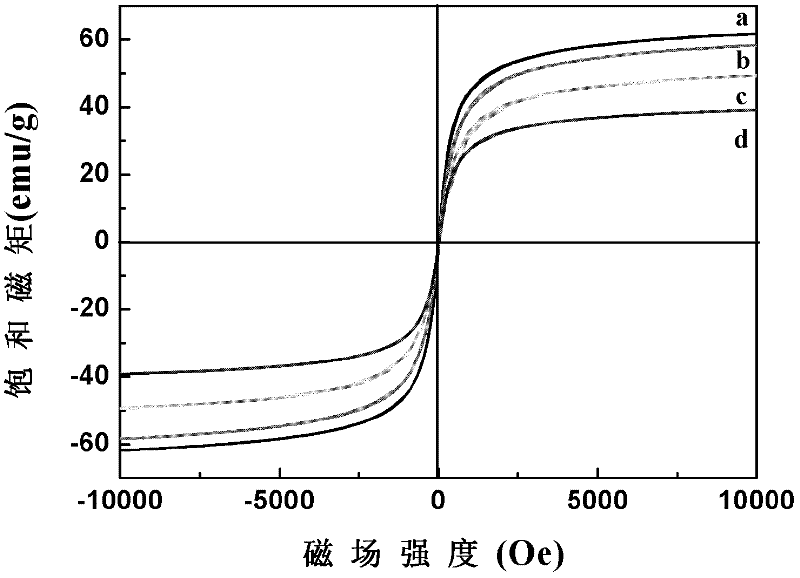
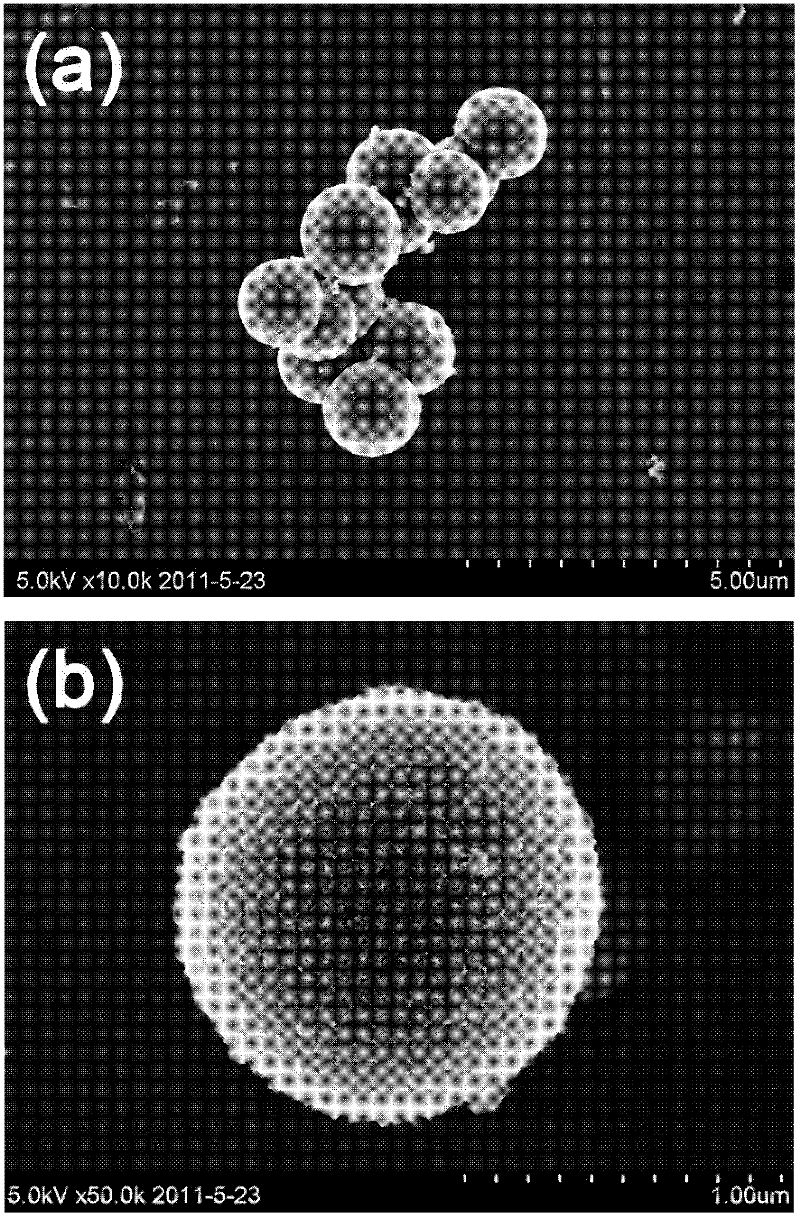
Preparation method for magnetic nanometer microballoon photocatalysis composite materials
A technology of magnetic nano and composite materials, applied in the field of preparation of photocatalytic composite materials, can solve the problems of accelerating the photocorrosion of magnetic materials, reducing the utilization of material light, and poor suspension of catalysts, so as to prevent photocorrosion and photocatalytic performance Good, high magnetic particle content effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) 24g FeCl 3 ·6H 2 O and 9.82 g FeCl 2 4H 2 O to mix, add 100mL deionized water and stir evenly, after mixing evenly, add 50mL ammonia water, add 3.76g oleic acid after 30 minutes, and continue the reaction for 1.5 hours. The whole process needs to be kept in an oil bath at 80°C. separated, washed three times with ethanol and deionized water, dried, and ground to obtain Fe 3 o 4 particles;
[0038] (2) Take the above Fe 3 o 4 For 0.1 g of particles, measure 3 mL of absolute ethanol (purity: 99.8%) and mix, weigh 0.09 g of TEOS, and add to the solution under ultrasonic treatment. After continuous sonication for 30 min, this solution was transferred to a vapor phase apparatus as the solid phase, and the liquid phase was 5 mL of distilled water and 0.1 mL of anhydrous ethylenediamine. The device was sealed and placed in an oven at 100°C for 12 hours. The solid phase product was washed several times with distilled water and ethanol, magnetically separated, and dr...
Embodiment 2
[0040] (1) 24g FeCl 3 ·6H 2 O and 9.82 g FeCl 2 4H 2 O to mix, add 100mL deionized water and stir evenly, after mixing evenly, add 50mL ammonia water, add 3.76g oleic acid after 30 minutes, and continue the reaction for 1.5 hours. The whole process needs to be kept in an oil bath at 80°C. separated, washed three times with ethanol and deionized water, dried, and ground to obtain Fe 3 o 4 particles;
[0041] (2) Take the above Fe 3 o 4 For 0.1 g of particles, measure 3 mL of absolute ethanol (purity: 99.8%) and mix, weigh 0.09 g of TEOS, and add to the solution under ultrasonic treatment. After continuous sonication for 30 min, this solution was transferred to a vapor phase apparatus as the solid phase, and the liquid phase was 5 mL of distilled water and 0.1 mL of anhydrous ethylenediamine. The device was sealed and placed in an oven at 100°C for 12 hours. The solid phase product was washed several times with distilled water and ethanol, magnetically separated, and dr...
Embodiment 3
[0044] (1) 24g FeCl 3 ·6H 2 O and 9.82 g FeCl 2 4H 2 O to mix, add 100mL deionized water and stir evenly, after mixing evenly, add 50mL ammonia water, add 3.76g oleic acid after 30 minutes, and continue the reaction for 1.5 hours. The whole process needs to be kept in an oil bath at 80°C. separated, washed three times with ethanol and deionized water, dried, and ground to obtain Fe 3 o 4 particles;
[0045] (2) Take the above Fe 3 o 4 For 0.1 g of particles, measure 3 mL of absolute ethanol (purity: 99.8%) and mix, weigh 0.09 g of TEOS, and add to the solution under ultrasonic treatment. After continuous sonication for 30 min, this solution was transferred to a vapor phase apparatus as the solid phase, and the liquid phase was 5 mL of distilled water and 0.1 mL of anhydrous ethylenediamine. The device was sealed and placed in an oven at 100°C for 12 hours. The solid phase product was washed several times with distilled water and ethanol, magnetically separated, and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com