Asymmetric bismuth catalysis system, preparation method and application thereof
A catalytic system and asymmetric technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems such as substrate limitations of asymmetric bismuth catalytic systems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
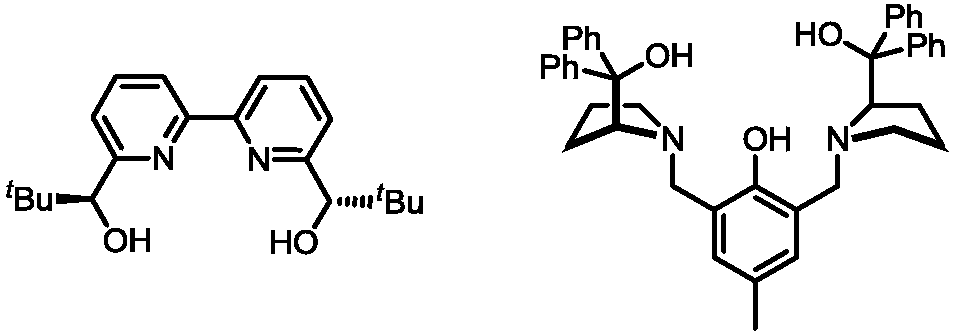
[0033]
[0034] With Bi(OAc) 3 / CPA catalytic system catalyzes the asymmetric allylation reaction of allylboronic acid pinacol ester (1.2equiv.) and isatin derivative ketimine (1.0equiv.) in ether solution at room temperature, the reaction The equation is as above. Bi(OAc) 3and chiral phosphoric acid (S)-A (the structure is shown in the dotted box on the right side) were added to the reaction tube, and then the substrate and the reaction solvent were added in sequence, stirred at room temperature, and the reaction was monitored by thin-layer chromatography. The consumption of catalyzer can be reduced to 1mol%, obtains 3-allyl-3-aminoindolinone with high yield and high enantioselectivity (99.5:0.5er (enantiomeric ratio, enantiomericratio)) up to 99% compound, and two drug molecules (+)-AG-041R and (-)-psychotriasine were obtained through a multi-step reaction.
Embodiment 2
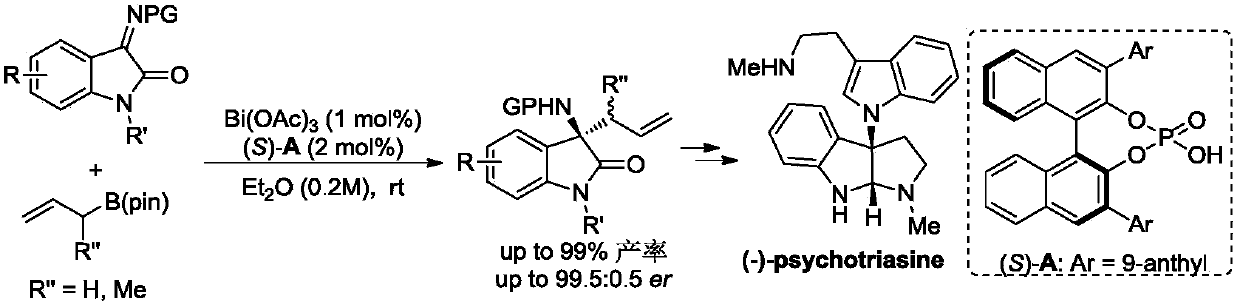
[0036]
[0037] With Bi(OAc) 3 The / CPA catalytic system is a catalyst that catalyzes the asymmetric allylation reaction of cyclic N-sulfonyl ketimine (1.0 equiv.) and allyl boric acid pinacol ester (1.2 equiv.) as substrates at room temperature , the reaction equation is as above. Bi(OAc) 3 and chiral phosphoric acid (S)-A were added to the reaction tube, and then the substrate and the reaction solvent were added in turn, stirred at room temperature, and the reaction was monitored by thin-layer chromatography. The amount of catalyst can be reduced to 1 mol%, and the target product is obtained in 91% yield and 94:6 enantioselectivity.
Embodiment 3
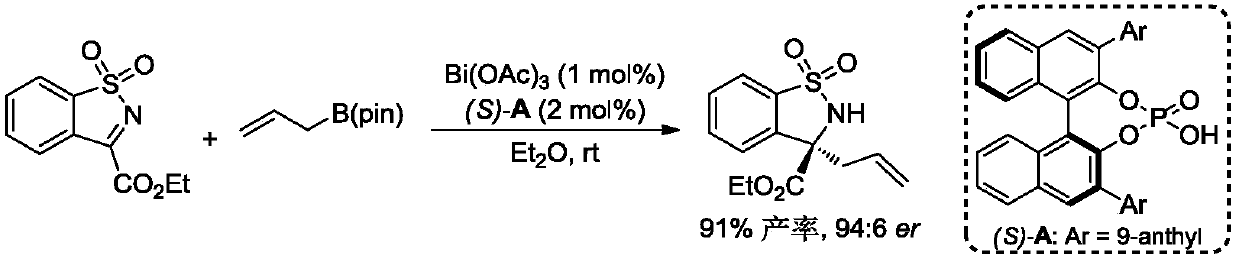
[0039]
[0040] With Bi(OAc) 3 / CPA catalytic system catalyzes N-1-methylene naphthalene protected indole quinone (1.0 equiv.) and allylboronic acid pinacol ester (1.2 equiv.) as substrates in cyclohexane solution at room temperature The asymmetric reaction, the reaction equation is as above. Bi(OAc) 3 and chiral phosphoric acid (S)-B were added to the reaction tube, and then the substrate and the reaction solvent were added in turn, stirred at room temperature, and the reaction was monitored by thin-layer chromatography. A series of 3-allyl-3-oxindolinone compounds were obtained with a high yield of 99% and high enantioselectivity (98:2er).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com