Method for preparing tert-butyl hydroperoxide employing biomimetic catalysis and isobutane oxidation
A technology of tert-butyl hydroperoxide and biomimetic catalysis of isobutane, which is applied to the preparation of peroxygen compounds, chemical instruments and methods, and the preparation of organic compounds, can solve the problems of poor selectivity of tert-butyl hydroperoxide and achieve The effect of avoiding environmental and safety problems, simple operation process, and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
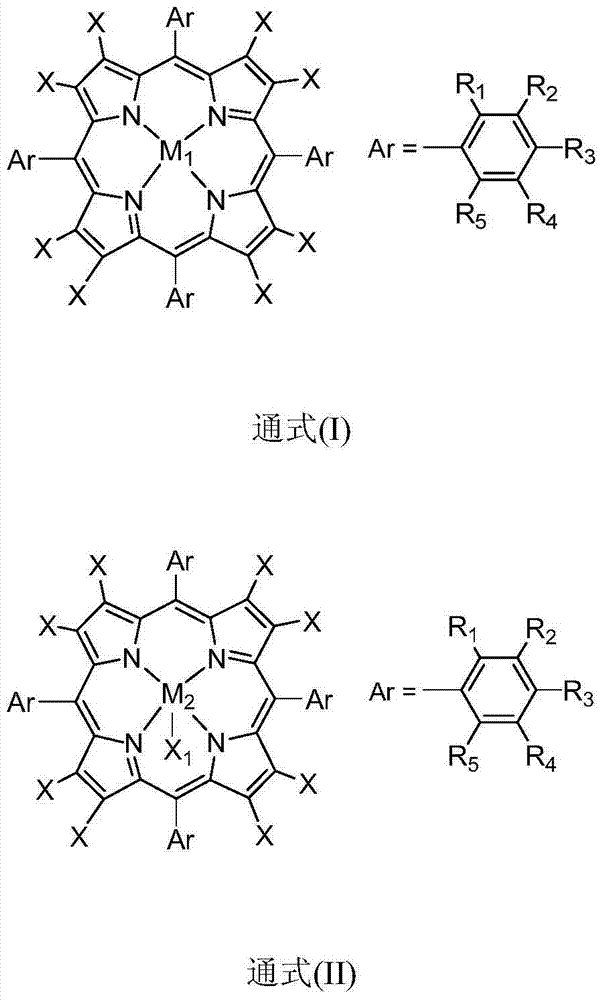
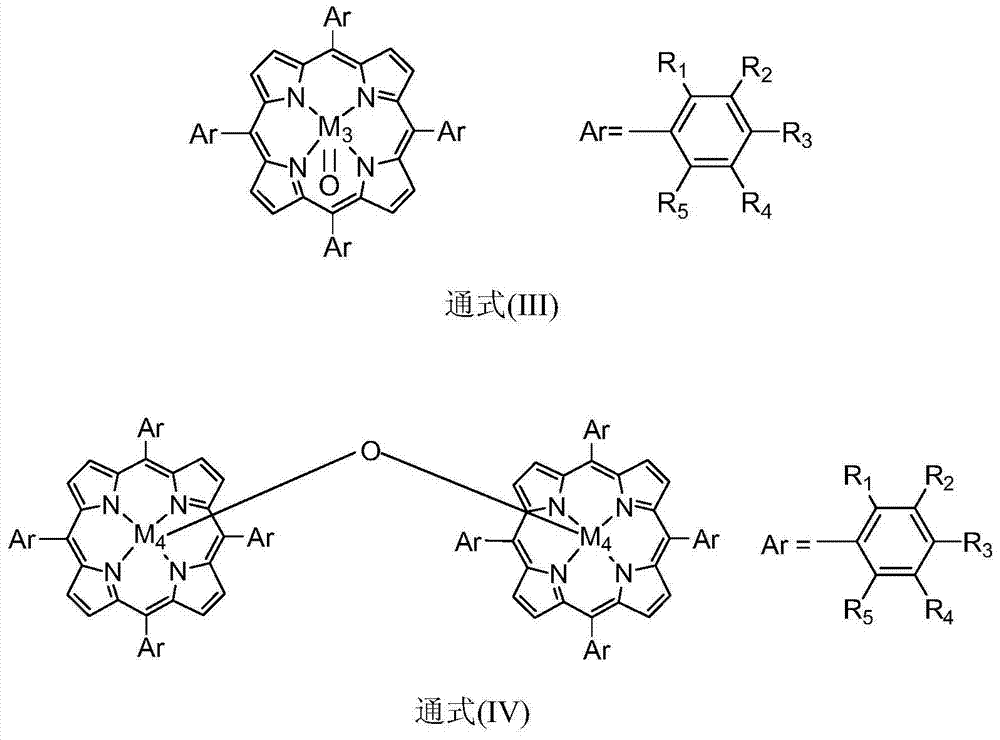
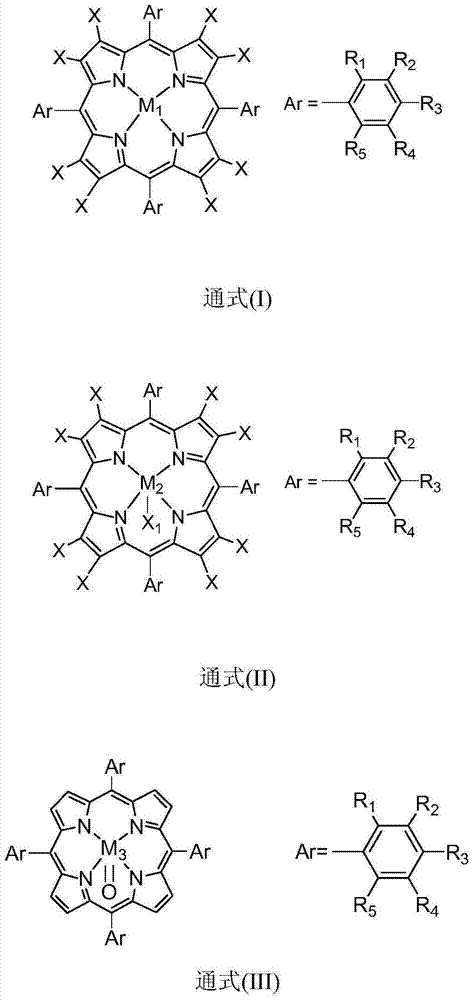
[0021] In 100mL polytetrafluoroethylene-lined stainless steel reactor, add 20mL containing 100ppm metalloporphyrin (M 1 =Mn,X=H,R 1 =R 2 =R 3 =R 4 =R 5 =H) in 1,2-dichloroethane solution, filled with 10mol, filled with oxygen, the reaction pressure was 0.3MPa, stirred at a temperature of 40°C, and reacted for 7h, detected by gas chromatography, isobutyl The conversion of alkanes was 17%, and the selectivity to tert-butyl hydroperoxide was 99%.
Embodiment 2
[0023] In 100mL polytetrafluoroethylene-lined stainless steel reactor, add 30mL containing 0.1ppm metalloporphyrin (M 1 =Co,X=H,R 1 = NO 2 , R 2 =R 3 =R 4 =R 5 =H) in the 1,2-dichloroethane solution, filled with 20mmol of isobutane, filled with oxygen, the reaction pressure was 3MPa, stirred at a temperature of 100°C, reacted for 7h, analyzed by gas chromatography , the conversion of isobutane was 15%, and the selectivity of tert-butyl hydroperoxide was 91%.
Embodiment 3
[0025] Add 30mL containing 10ppm metalloporphyrin (M 2 =Fe,X=F,R 1 =R 2 =R 3 =R 4 =R 5 =H,X 1 Pyridine) in the acetonitrile solution, filled with 20mmol isobutane, filled with oxygen, the reaction pressure is 2MPa, stirred at a temperature of 60 ° C, after 7 hours of reaction, through gas chromatography analysis, the conversion rate of isobutane was 25 %, the selectivity of tert-butyl hydroperoxide is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com