Method for preparing 1-amino-2-cyano cyclopentene
A technology of cyanocyclopentene and amino, which is applied in the preparation of carboxylic acid nitrile, chemical instruments and methods, preparation of ammonia-carboxylic acid reaction, etc. It can solve the problem of difficult to obtain pure adipamide and difficult to buy ACCP finished products , less ACCP and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
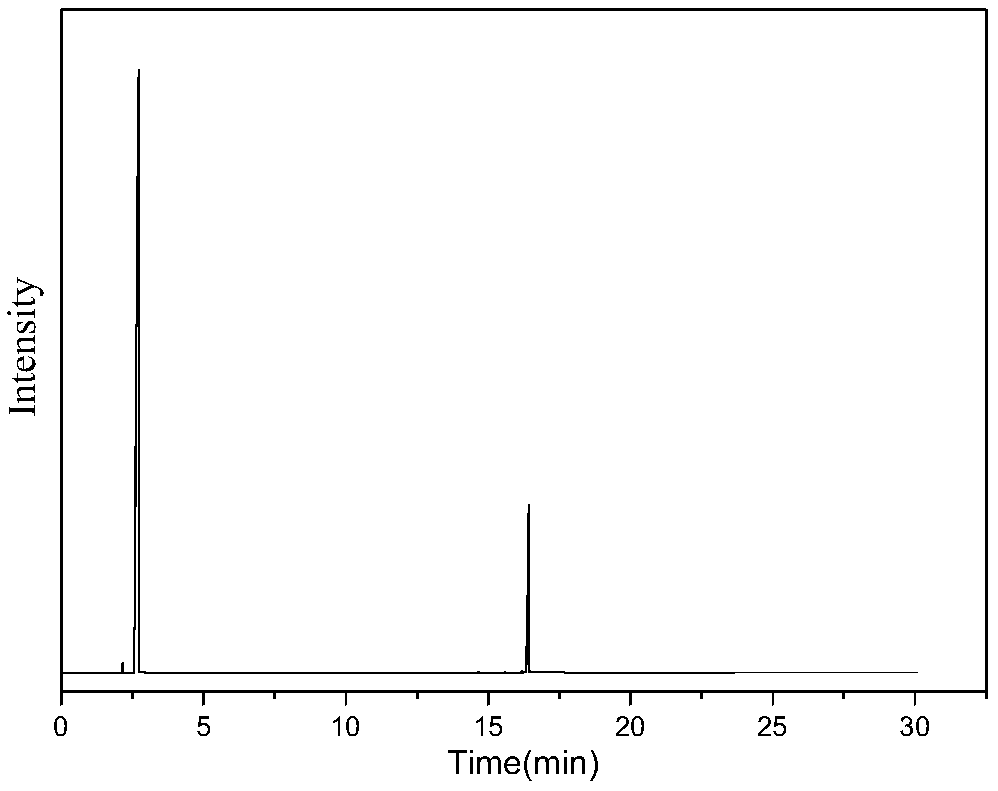
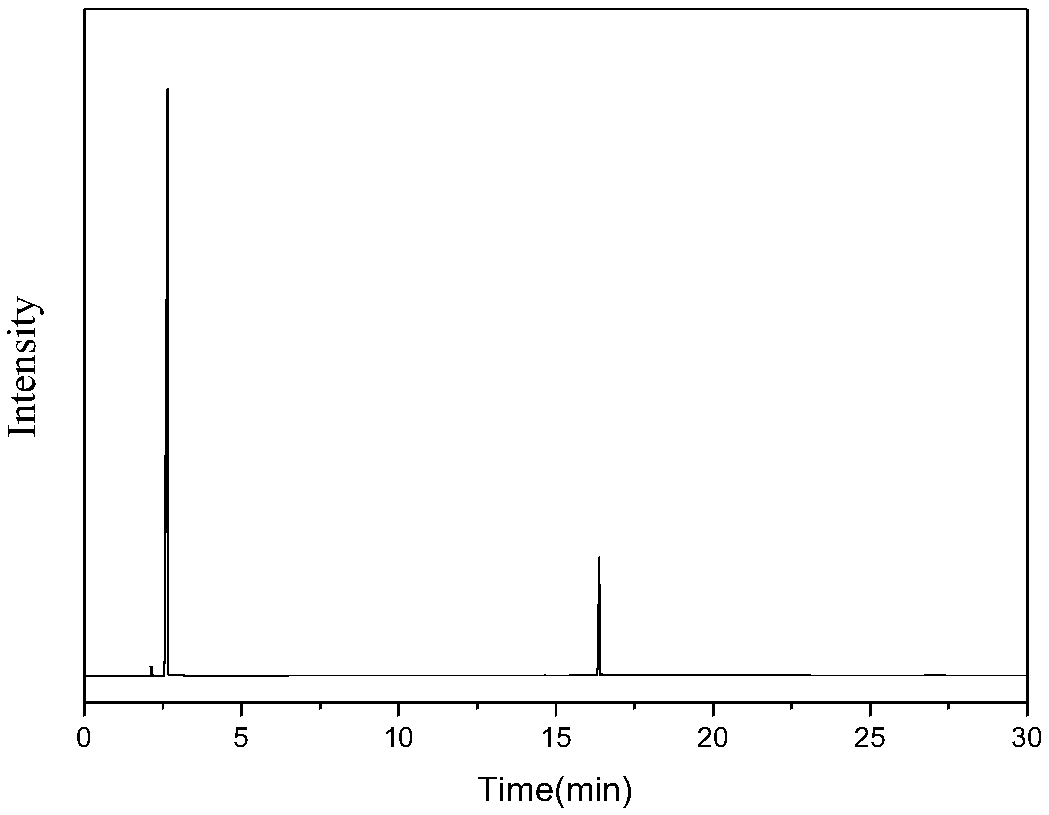
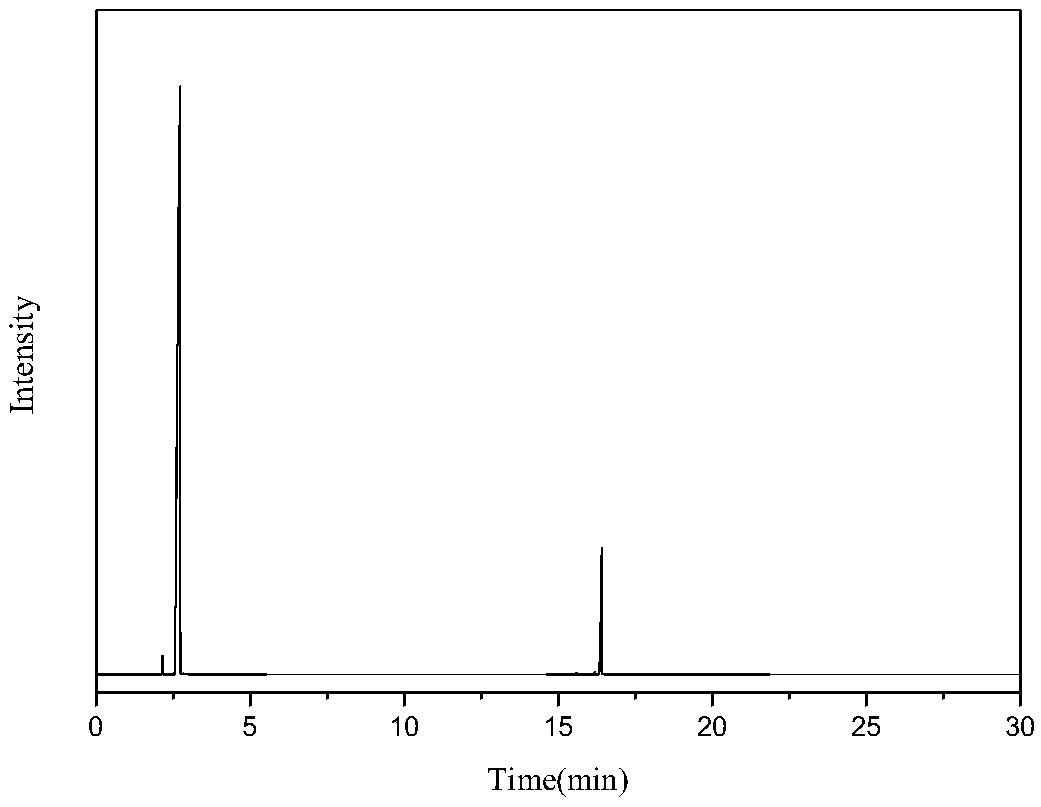
[0032] Mix 0.5g / min dimethyl adipate with 1SLM ammonia and enter the fluidized bed reactor to react with a solid acid catalyst. The catalyst is alumina, the reaction temperature is 330°C, the reaction pressure is 0.1mpa, and the catalyst loading is 10g, and the mass space velocity of dimethyl adipate is 3h -1 , the contact time was 1s, the reaction was stopped after 10 hours of reaction, and the reaction product was condensed and collected to obtain 358g of the product. The main components included adiponitrile, ACCP, methanol and water, wherein the content of ACCP was 13.03g, and the content of adiponitrile was 186g. Then the reaction product was rectified under reduced pressure at 5000 Pa to obtain 87 g of a fraction at 170-180° C., which was mainly a mixture of ACCP and adiponitrile. Put the fraction at 5°C for 2 hours, a large amount of ACCP precipitated, filtered it to obtain 10.5 g of crude ACCP, completely dissolved ACCP with 70°C benzene, then cooled to 10°C, filtered ...
Embodiment 2
[0034] Mix 0.3g / min dimethyl adipate and 1.5SLM ammonia gas into a fixed bed reactor to react with a magnesium phosphate catalyst, the catalyst is magnesium oxide, the reaction temperature is 350°C, the reaction pressure is 0.1mpa, and the catalyst loading is 10g, and the mass space velocity of dimethyl adipate is 1.8h -1 , the contact time was 0.6s, and the reaction was stopped after 24 hours of reaction. The ammonated product of dimethyl adipate was condensed and collected to obtain 515g of the product, the main components of which were adiponitrile, ACCP, methanol and water, wherein the ACCP content was 26.8g, The dinitrile content was 267 g. The reaction product was then subjected to rectification under reduced pressure at 5000 Pa to obtain 105 g of cuts at 170-180° C., and the cuts were mainly a mixture of ACCP and adiponitrile. Put the fraction at 5°C for 2 hours, a large amount of ACCP precipitated, filtered it to obtain 23.5g of crude ACCP, completely dissolved ACCP w...
Embodiment 3
[0036] Mix 0.3g / min dimethyl adipate and 2SLM ammonia into a fixed bed reactor to react with a solid acid catalyst, the catalyst is magnesium phosphate, the reaction temperature is 400°C, the reaction pressure is 0.5mpa, and the catalyst loading is 18g, dimethyl adipate mass space velocity 1h -1 , the contact time was 0.4s, and the reaction was stopped after 10 hours of reaction. The ammonated product of dimethyl adipate was condensed and collected to obtain 215g of the product, the main components of which were adiponitrile, ACCP, methanol and water, wherein the content of ACCP was 6.7g, The dinitrile content was 111 g. The reaction product was then subjected to rectification under reduced pressure at 5000 Pa to obtain 26 g of cuts at 170-180° C., which were mainly ACCP and adiponitrile mixtures. Put the fraction at 5°C for 2 hours, a large amount of ACCP precipitated, filtered it to obtain 5.8 g of crude ACCP, completely dissolved ACCP with xylene at 100°C, then cooled to 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com