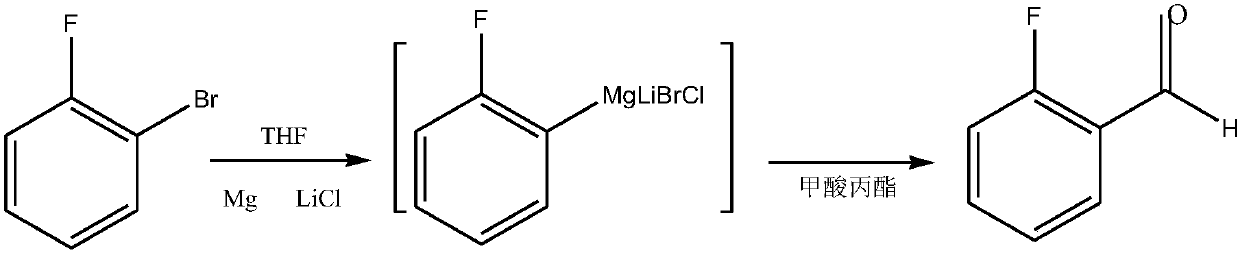
Preparation method of 2-fluorobenzaldehyde
A technology of fluorobenzaldehyde and benzaldehyde, which is applied in the field of preparation of o-fluorobenzaldehyde, can solve the problems of large consumption of catalyst, environmental pollution, reduction of the scope of use of o-fluorobenzaldehyde and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A preparation method of o-fluorobenzaldehyde, comprising the following steps:
[0035] (1) 1.25 moles of propyl formate were dissolved in 150 ml of anhydrous tetrahydrofuran to obtain solution A.
[0036] (2) Solution B in which 1 mole of o-fluorobromobenzene is dissolved in 200 ml of anhydrous tetrahydrofuran.
[0037] (3) Put 1 mole of anhydrous lithium chloride and 1.15 moles of magnesium into a reaction flask, add 50 ml of anhydrous tetrahydrofuran, heat to a slight boil (about 65 ° C), and drop 2 ml of dibromoethane to initiate the reaction.
[0038] (4) Under reflux, solution B was added dropwise into the above-mentioned reaction flask, and the temperature was controlled at 63-67°C during the process.
[0039] (5) After feeding, cool down to 20-30°C, add solution 1 dropwise; react for 1 hour after feeding.
[0040] (6) Cool down to below 10°C, quench with dilute hydrochloric acid, post-process, and refine by vacuum distillation.
[0041] (7) 113.5 g of o-fluoro...
Embodiment 2
[0043] A preparation method of o-fluorobenzaldehyde, comprising the following steps:
[0044] (1) 1 mole of propyl formate was dissolved in 100 ml of anhydrous tetrahydrofuran to obtain solution A.
[0045] (2) Solution B in which 1 mole of o-fluorobromobenzene is dissolved in 150 ml of anhydrous tetrahydrofuran.
[0046] (3) Put 1 mole of anhydrous lithium chloride and 1.15 moles of magnesium into a reaction flask, add 30 ml of anhydrous tetrahydrofuran, heat to a slight boil (about 65° C.), and drop 2 ml of dibromoethane to initiate the reaction.
[0047] (4) Under reflux, solution B was added dropwise into the above-mentioned reaction flask, and the temperature was controlled at 63-67°C during the process.
[0048] (5) After feeding, cool down to 20-30°C, add solution 1 dropwise; react for 0.5 hours after feeding.
[0049] (6) Cool down to below 10°C, quench with dilute hydrochloric acid, post-process, and refine by vacuum distillation.
[0050] (7) 110 g of o-fluorobenz...
Embodiment 3
[0052] A preparation method of o-fluorobenzaldehyde, comprising the following steps:
[0053] (1) 1.5 moles of propyl formate were dissolved in 200 ml of anhydrous tetrahydrofuran to obtain solution A.
[0054] (2) Solution B in which 1 mole of o-fluorobromobenzene is dissolved in 250 ml of anhydrous tetrahydrofuran.
[0055] (3) Put 1 mole of anhydrous lithium chloride and 1.15 moles of magnesium into a reaction flask, add 70 ml of anhydrous tetrahydrofuran, heat to a slight boil (about 65° C.), and drop 2 ml of dibromoethane to initiate the reaction.
[0056] (4) Under reflux, solution B was added dropwise into the above-mentioned reaction flask, and the temperature was controlled at 63-67°C during the process.
[0057] (5) After feeding, cool down to 20-30°C, add solution 1 dropwise; react for 1.5 hours after feeding.
[0058] (6) Cool down to below 10°C, quench with dilute hydrochloric acid, post-process, and refine by vacuum distillation.
[0059] (7) 115 g of o-fluoro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com