Azo compound, polymer, preparation method and use
A technology of azo compounds and compounds, applied in the field of eye medical devices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
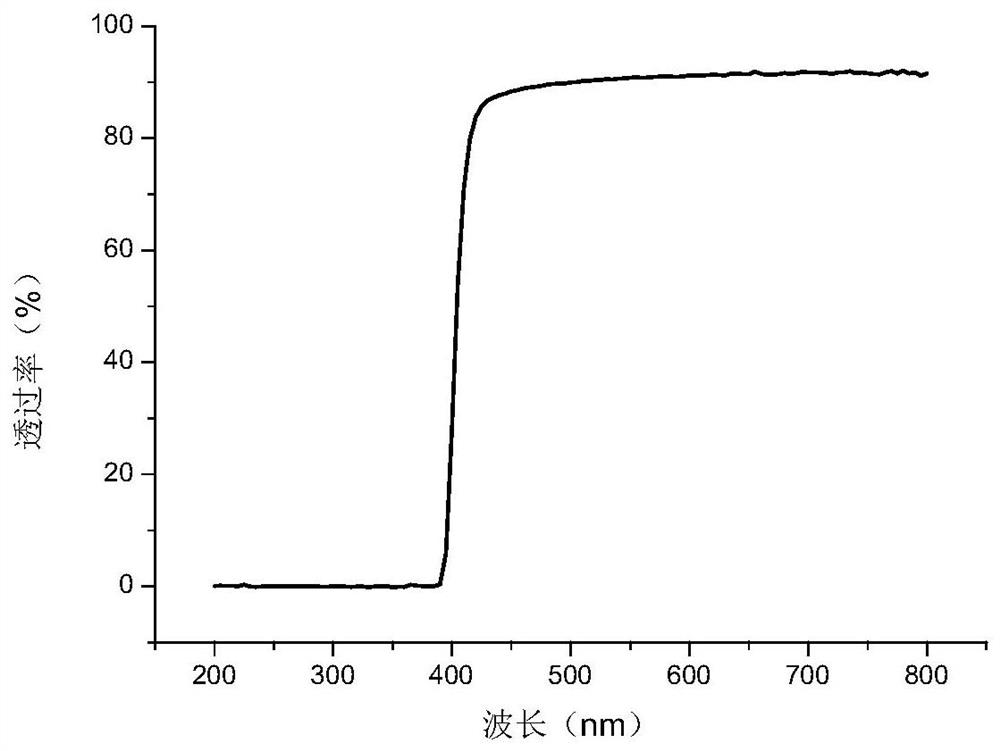
[0099] The preparation of embodiment 1 azo compound
[0100] The following describes the azo compound according to the synthetic route: the preparation of 1,3-bis(4-(4'-hydroxyazophenyl)oxy)-2-propyl methacrylate (chemical formula is as follows):
[0101]
[0102] (1) Preparation of 1,3-bis(4-nitrophenoxy)-2-propanol:
[0103] 4-Nitrophenol (11.2g, 81.2mmol), potassium carbonate (11.2g, 81.2mmol) and absolute ethanol (200mL) were successively added into a three-necked flask, and the mixture was refluxed and stirred for 1h, and then 1 , 3-Dibromo-2-propanol (4.4g, 20.2mmol), and stirring was continued for 24h. The reaction solution was poured into water (500mL), stirred for 0.5h and then filtered. The filter cake was washed with aqueous sodium hydroxide solution (10%wt, 200mL) and water (300mL), and the collected solid was vacuum-dried at 50°C for 5h to obtain A total of 4.8 g of light-colored solid 1,3-bis(4'-nitrophenoxy)-2-propanol was obtained, and the yield was 68%....
Embodiment 2
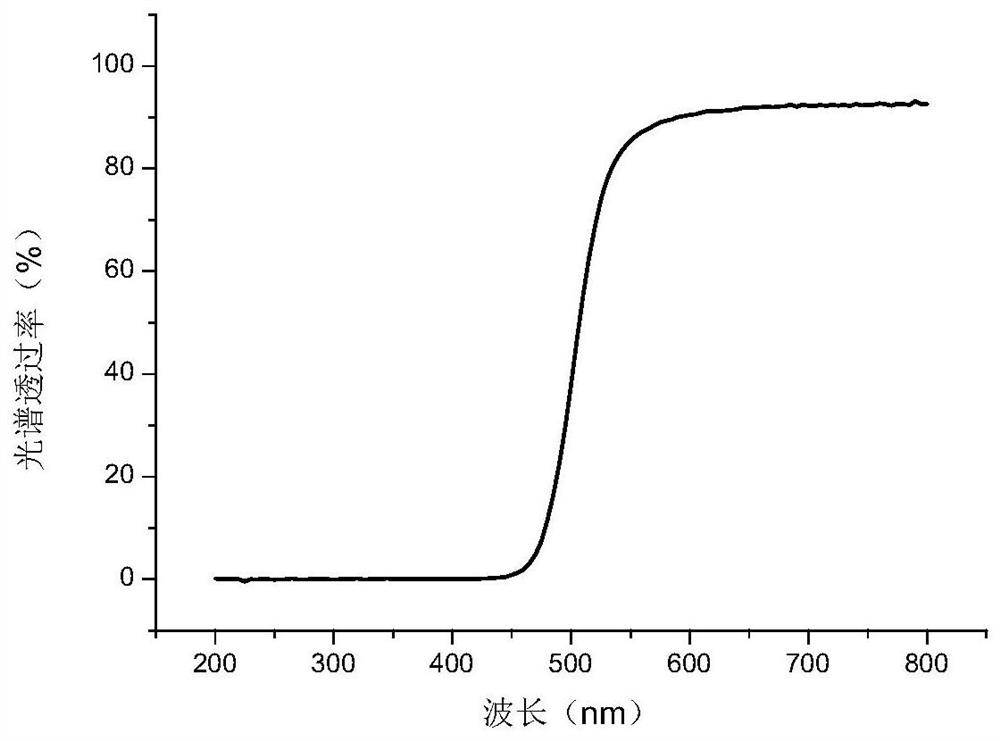
[0125] The preparation of embodiment 2 azo compounds
[0126] The following describes the preparation of the azo compound: 1,3-bis(4-(2',4'-dihydroxyazobenzene)oxy)-2-propyl methacrylate (chemical formula as follows) according to the synthetic route:
[0127]
[0128] The remaining steps are the same as in Example 1, except that in step (6), 1,3-bis(4-aminophenoxy)-2-propylmethacrylate (1.2g , 3.5mmol), potassium bromide (2.99g, 25.6mmol), a mixed solution (20mL) of concentrated hydrochloric acid (2.55g, 25.6mmol) and acetone / water (volume ratio 1:1), and the mixture was placed in a -5°C low-temperature bath When the internal temperature reached below 0°C, sodium nitrite (0.485g, 7.0mmol) was dissolved in water (10mL), slowly added dropwise to the above reaction solution, and continued to stir for 0.5h. Dissolve resorcinol (0.88g, 8.0mmol) and sodium hydroxide (0.63g, 15.8mmol) in water (20mL), and add dropwise to the above-mentioned diazonium salt solution, and keep the s...
Embodiment 3
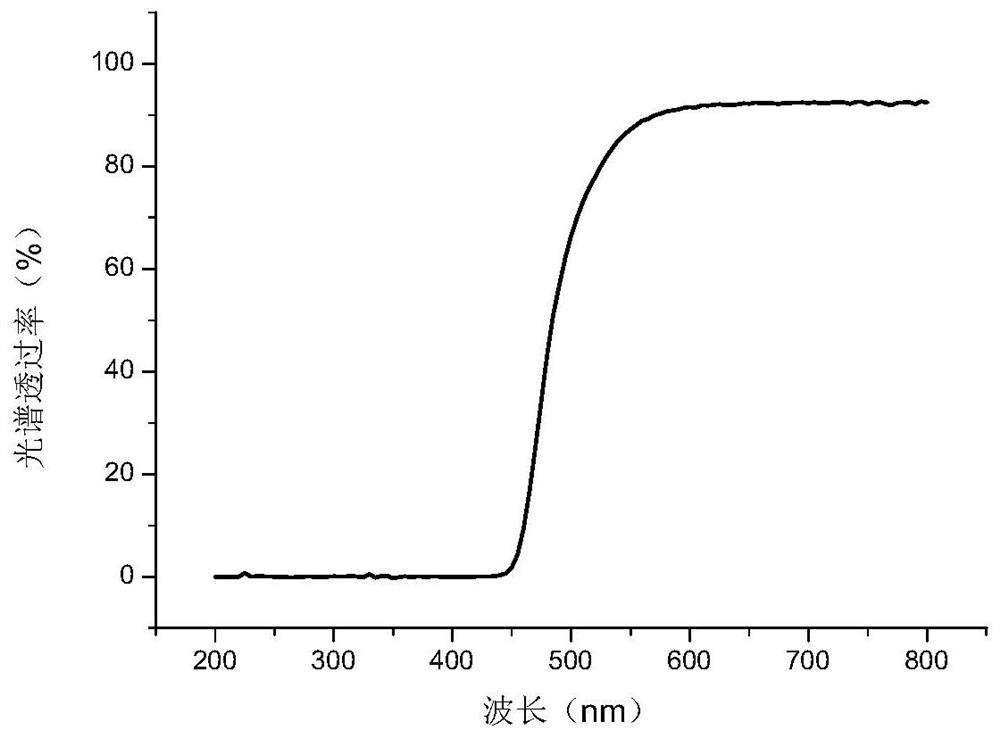
[0131] The preparation of embodiment 3 azo compounds
[0132] The following describes the preparation of the azo compound 1,3-bis(4-(2'-methyl-4'-hydroxyazobenzene)oxy)-2-propyl methacrylate (chemical formula as follows) according to the synthetic route :
[0133]
[0134] The remaining steps are the same as in Example 1, except that in step (6), 1,3-bis(4-aminophenoxy)-2-propylmethacrylate (2.0g , 5.83mmol), a mixed solution (20mL) of potassium bromide (1.2g, 10.1mmol), concentrated hydrochloric acid (4.0g, 39.5mmol) and acetone / water (v / v=1:1), and the mixture was stored at -5°C Stir in a low-temperature bath, and when the internal temperature reaches below 0°C, dissolve sodium nitrite (0.97g, 14.0mmol) in water (10mL), slowly add dropwise to the above reaction solution, and continue stirring for 0.5h. Dissolve m-cresol (1.26g, 9.20mmol), sodium hydroxide (0.93g, 23.3mmol), sodium carbonate (1.23g, 11.6mmol) in water (20mL), and add dropwise to the above diazonium salt ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com