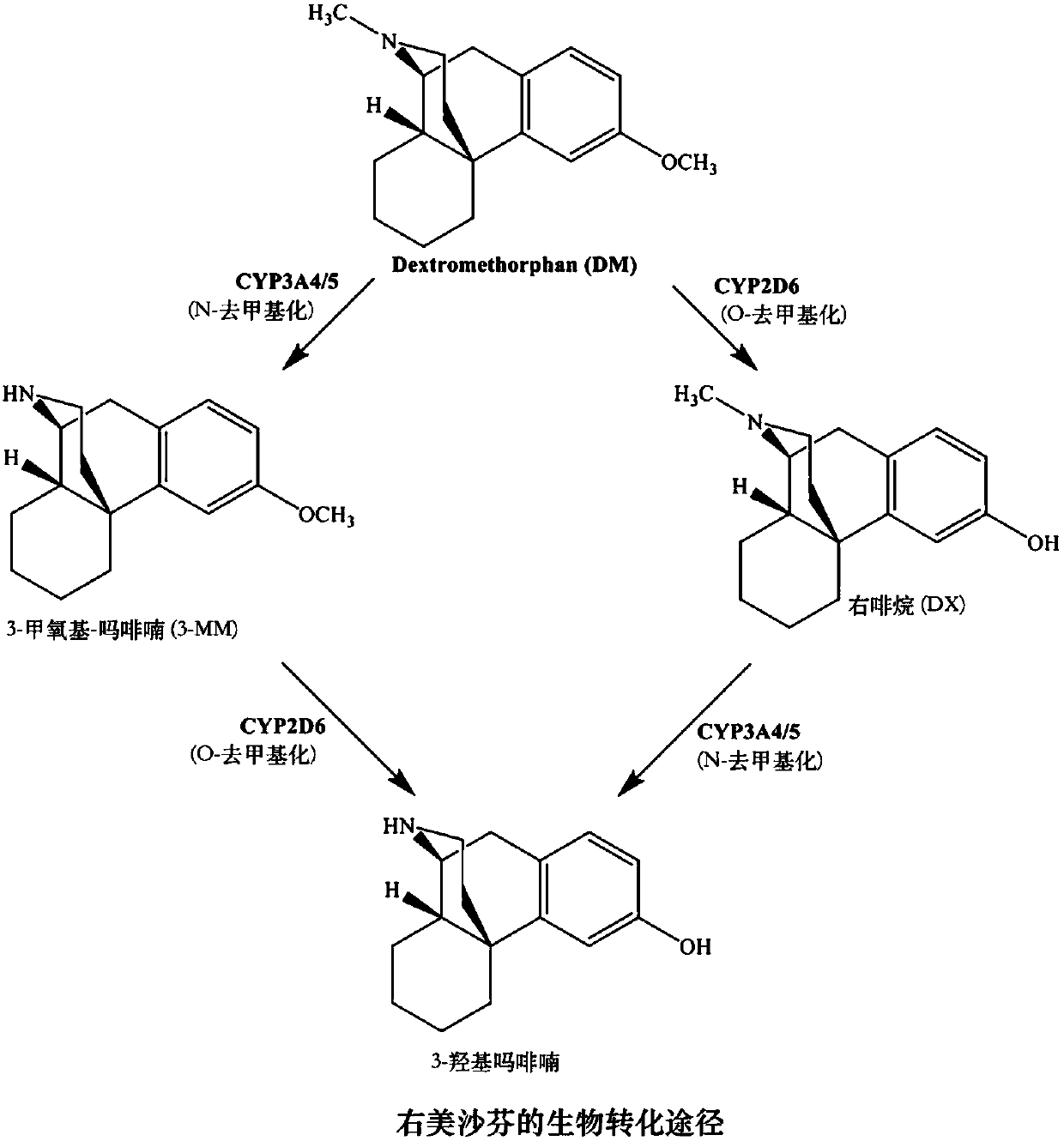
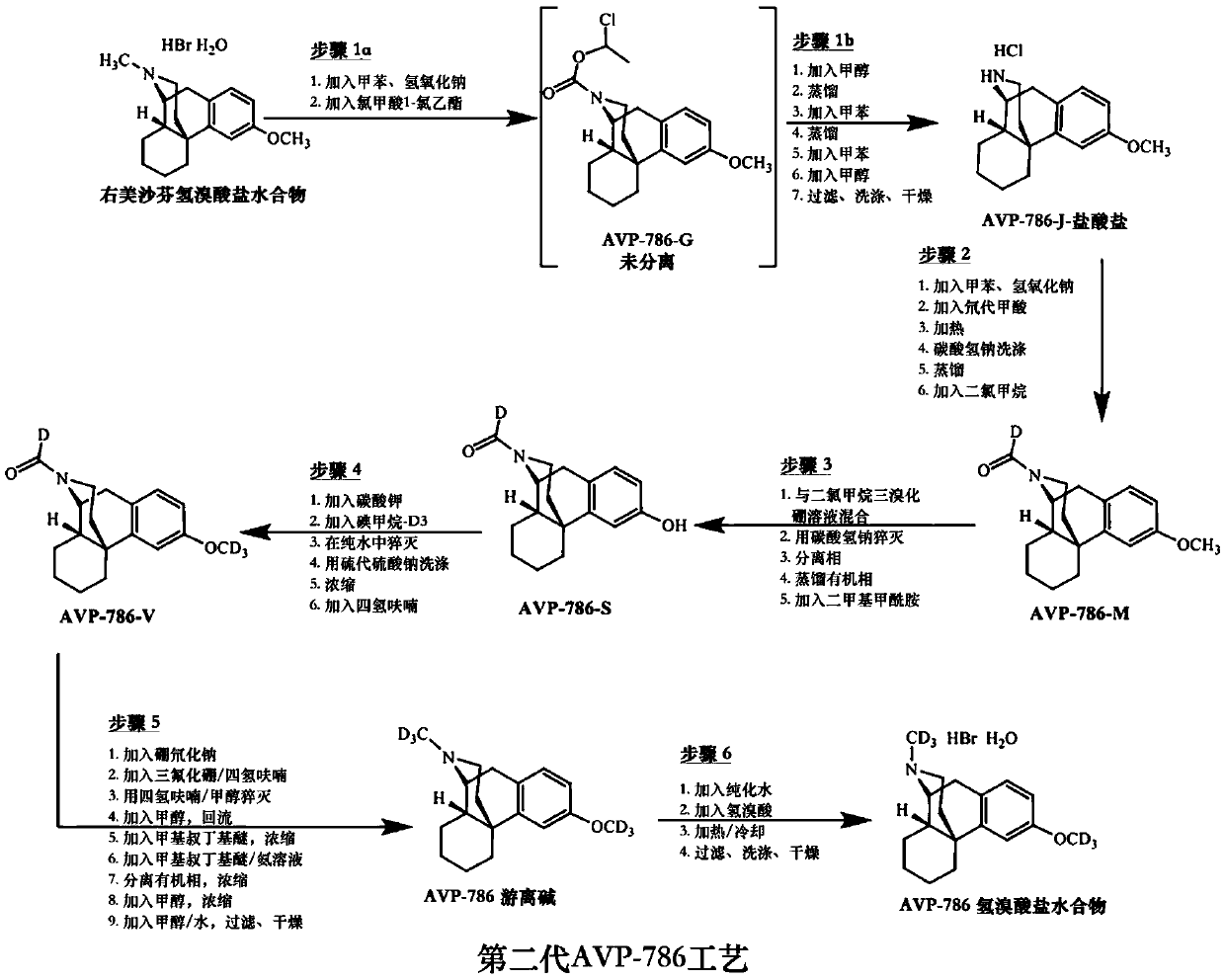
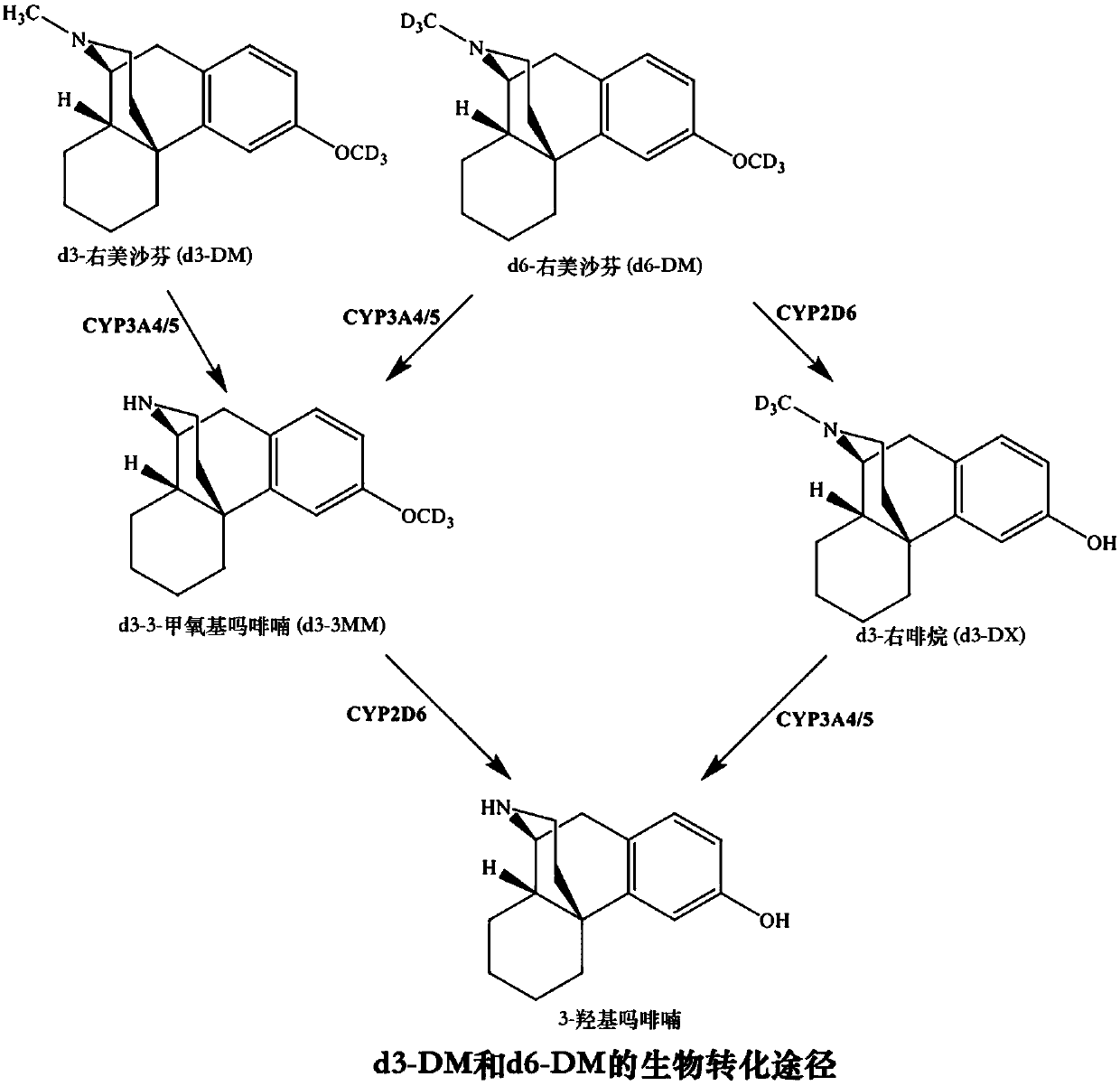
Methods for the synthesis of deuterated dextromethorphan
A dextromethorphan and deuterated technology, applied in the field of synthesis of deuterated dextromethorphan, can solve problems such as adverse events, slow metabolism, prolonged side effects, etc., achieve improved separation yield and purity, increase yield and purity, simplify The effect of the supply chain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0214] In one embodiment, such methods of preparation include the step of bringing into association the molecule to be administered with ingredients such as a pharmaceutically acceptable carrier. In one embodiment, the compositions are prepared by uniformly and intimately bringing into association the active ingredient with one or more liquid carriers, liposomes or finely divided solid carriers, and then, if necessary, shaping the product.
[0215] In one embodiment, the compound is administered orally. Compositions of the present invention suitable for oral administration may be presented as discrete units such as, but not limited to, capsules, sachets or tablets each containing a predetermined amount of active ingredient; powder or granules; solutions in aqueous or non-aqueous liquids or Suspension; oil-in-water liquid emulsion; water-in-oil liquid emulsion; packaged in liposomes; or as a bolus injection, etc. In one embodiment, soft gelatin capsules are used to contain thi...
Embodiment 1
[0295] Synthesis of compounds of formula (I). Each reaction and numbered intermediate described below refers to the corresponding reaction and intermediate in Scheme 1 above.
[0296] Reaction (i) (intermediate d6-DM-J hydrochloride): a solution of dextromethorphan hydrobromide (1.1 kg, 2.97 mol, 1.0 equiv) in toluene (13.2 L, 12 v) was mixed with aqueous sodium hydroxide ( 1M concentration) mixed. After stirring, the organic phase was separated, washed with water to neutral pH, and dried azeotropically. 1-Chloroethyl chloroformate (0.552 kg, 3.86 mol, 1.3 equiv) was added to the solution, and the mixture was stirred until conversion > 93%. The mixture was heated to 65-70°C and methanol (2.09kg, 1.9kg / kg) was added slowly. Continue heating until the reaction conversion is ≥98%. The reaction mixture was cooled and concentrated under vacuum until the methanol level dropped to NMT 100 ppm. Toluene (4.0 L, 3.6 v) was added and the mixture was again concentrated under vacuum. ...
Embodiment 2
[0301] Synthesis of compounds of formula (IV). Each reaction and numbered intermediate described below refers to the corresponding reaction and intermediate in Scheme 3 above.
[0302] Reaction (v) (d6-DM hydrobromide monohydrate): All solutions were filtered using a 0.45 μm filter and all manipulations were performed in a class 100,000 clean room. Purified water (1.1 L, 4v) and d6-DM free base (277.4 g, 1.00 mol) were mixed in the reactor. A solution of hydrobromic acid (48% strength, 177.0 g, 1.05 mol) was added, followed by purified water (0.28 L, 1 v). The resulting mixture was heated to 65-70°C until a solution formed, then cooled to effect crystallization. The mixture was heated and cooled for several cycles to optimize particle size, then cooled to ambient temperature. The product was isolated by filtration, washed with pure water, and dried at 40-45° C. to obtain the compound of formula (IV). The compound of formula (IV) was obtained with a yield of 85.0% and a pur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com