Preparation method for esomeprazole
A technology of esomeprazole and methoxybenzimidazole, which is applied in the field of high-efficiency enzyme catalyst preparation of esomeprazole, can solve the problems of low selectivity, difficulty in realizing industrial production, complicated operation, etc., and achieve the reduction of impurity nitrogen Oxides, improved yield and purity, and ease of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
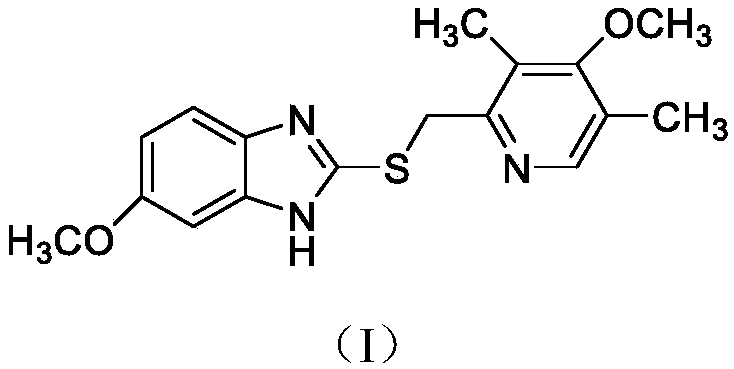
[0052] (S)-5-methoxy-2-[[4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole (Exome Prazole) preparation
[0053] Get 100 grams of methanol and pour it into a 500 milliliter three-necked flask, add 8.8 grams (220 mmol) of sodium hydroxide, then put 23.0 grams (130 mmol) of 2-mercapto-5-methoxybenzimidazole into the above-mentioned sodium hydroxide Stir in the methanol solution to make it dissolve, then add 24.0 g (110 mmol) 2-chloromethyl-4-methoxy-3,5-lutidine hydrochloride into the reaction solution, after the addition, heat up Stir the reaction at 50°C for 4 hours, and determine the end point of the reaction by TLC (the developer is V dichloromethane: V methanol = 30:1). After the reaction is completed, add 200 grams of drinking water dropwise to crystallize overnight, then filter, wash and dry to obtain 34.1 g of the compound having the structure of formula (I).
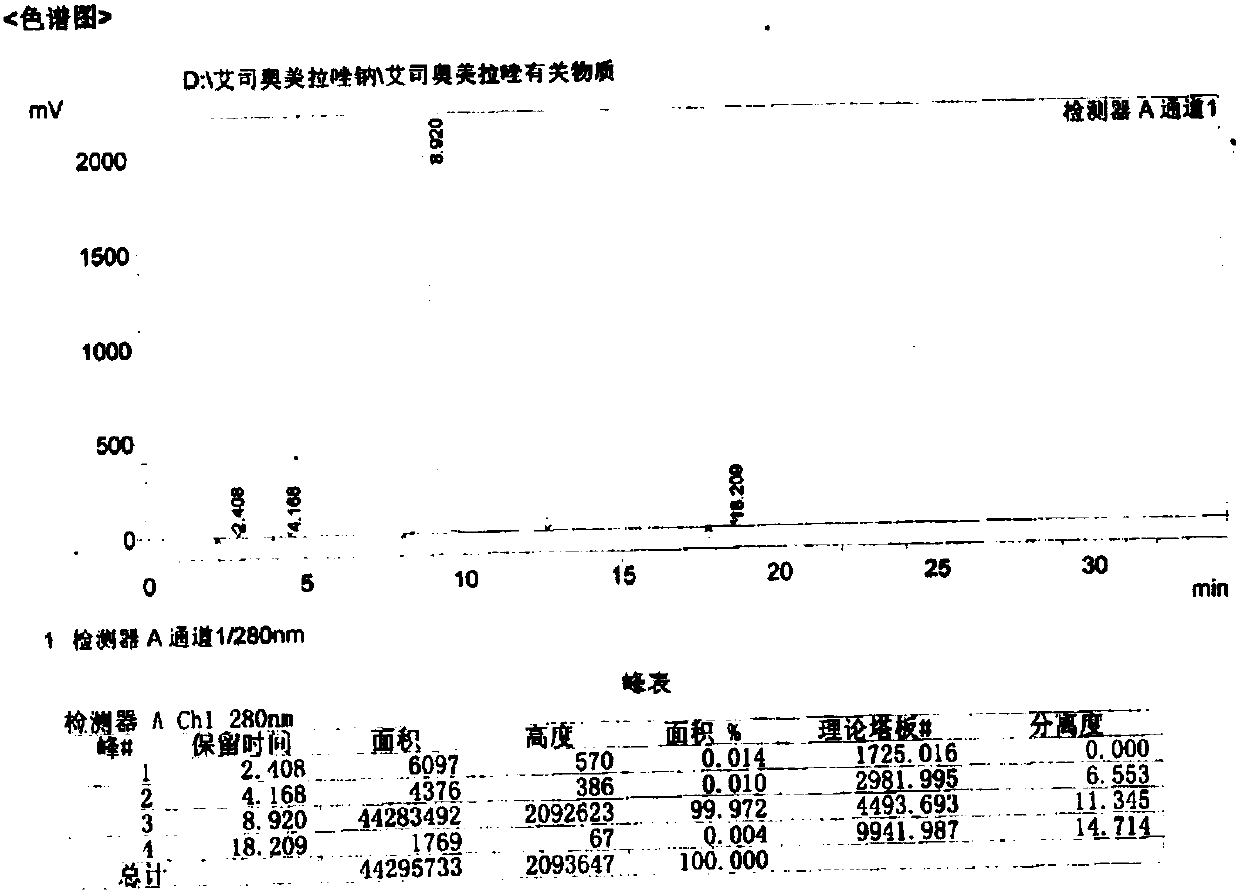
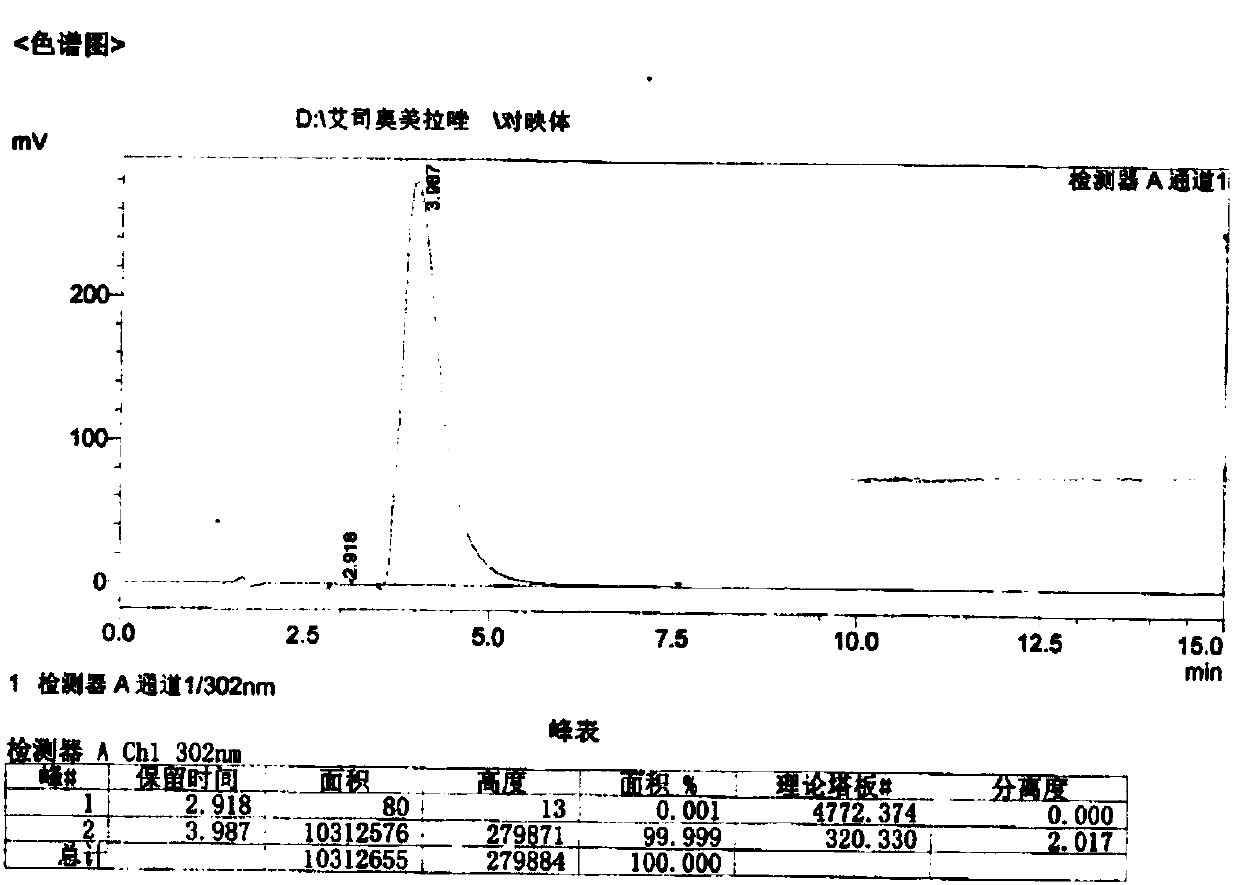
[0054] The purity of the compound with the structure of formula (I) measured by HPLC is 99.8%...
Embodiment 2
[0061] (S)-5-methoxy-2-[[4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole potassium (Esso Meprazole potassium) preparation
[0062] Get 100 grams of ethanol and pour it into a 500 milliliter three-necked bottle, add 12.32 grams (220 mmol) of potassium hydroxide, then put 23.0 grams (130 mmol) of 2-mercapto-5-methoxybenzimidazole into the above potassium hydroxide Stir in the ethanol solution to dissolve it, then add 24.0 g (110 mmol) 2-chloromethyl-4-methoxy-3,5-lutidine hydrochloride into the reaction solution, after the addition, continue React at 40°C for 3 hours, TLC determines the reaction end point (developing agent is V dichloromethane: V methanol = 30: 1), after the reaction is completed, add 200 grams of drinking water dropwise to crystallize overnight, then filter, wash and dry to obtain the product with 33.7 grams of the compound of formula (I).
[0063] The purity of the compound with the structure of formula (I) measured by HPLC is 99.8%
[00...
Embodiment 3
[0070] (S)-5-methoxy-2-[[4-methoxy-3,5-dimethyl-2-pyridyl)methyl]sulfinyl]-1H-benzimidazole potassium (Esso Meprazole potassium) preparation
[0071] Get 300 grams of acetone and pour it into a 1000 milliliter three-necked bottle, add 69.96 grams (660 mmol) of sodium carbonate, then drop 69.0 grams (390 mmol) of 2-mercapto-5-methoxybenzimidazole into the above-mentioned sodium carbonate acetone solution Stir in medium to dissolve it, then add 72.0 grams (330 mmol) of 2-chloromethyl-4-methoxy-3,5-lutidine hydrochloride into the reaction solution, after the addition, continue at 55 ℃ for 5 hours, TLC to determine the reaction end point (developing agent is V dichloromethane: V methanol = 30: 1), after the completion of the reaction, add 600 grams of drinking water dropwise to crystallize overnight, then filter, wash and dry to obtain formula ( 100.2 grams of the compound of structure I).
[0072] The purity of the compound with the structure of formula (I) measured by HPLC is ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com