Polyamide acid, polyamide acid solution, polyimide, polyimide film, laminate, flexible device, and method of manufacturing polyimide film
A technology of polyimide film and polyamic acid, which is applied in the field of polyamic acid solution, polyimide film, polyimide, and polyamic acid, can solve problems such as component damage and substrate warping, and achieve Excellent thermal dimensional stability and transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0039] The preparation method of the polyamic acid solution containing imidazoles is not particularly limited. Imidazoles may be added to a polyamic acid solution obtained by polymerization of tetracarboxylic dianhydride and diamine in an organic solvent, or imidazoles may be added to a solution before or during the polymerization reaction. When imidazoles are contained in a reaction system, tetracarboxylic dianhydride ring-opens and the reactivity with diamine may fall. Therefore, the method of adding imidazoles to the polyamic-acid solution obtained by superposition|polymerization of tetracarboxylic dianhydride and diamine is preferable from a viewpoint of controlling the molecular weight of polyamic acid. The imidazoles may be directly added to the polyamic acid, or the imidazoles previously mixed with a solvent may be added to the polyamic acid.
[0040] Various organic or inorganic low-molecular or high-molecular compounds may be compounded in order to impart processabil...
Embodiment 1
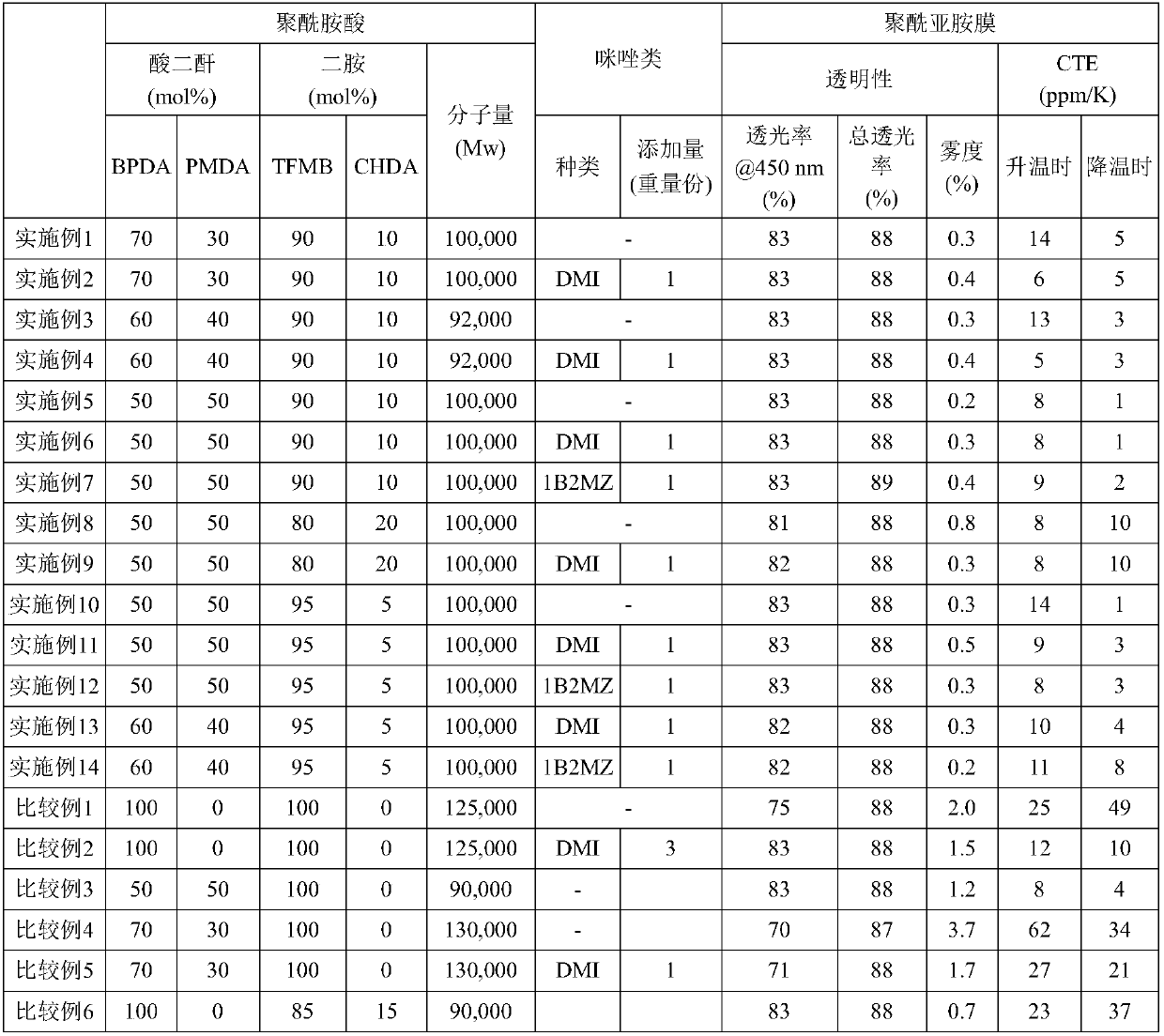
[0070] Add 400.00 g of N-methyl-2-pyrrolidone (hereinafter referred to as NMP) and 50.47 g of TFMB to a 2 L separable glass flask equipped with a stirrer equipped with a stainless steel stirring rod and a nitrogen gas introduction tube, and stir And after making it melt|dissolve, CHDA2.00g was added, it stirred, and it melt|dissolved. Stirring the solution, 36.07 g of BPDA and 11.46 g of PMDA were added sequentially, and it stirred for 24 hours, and obtained the polyamic-acid solution. The addition concentration of the diamine component and the tetracarboxylic dianhydride component in this reaction solution was 20.0 weight% with respect to the reaction solution whole quantity.
Embodiment 2
[0072] To 500 g of a 20.0% by weight polyamic acid solution synthesized in the same manner as in Example 1, 1 g of 1,2-dimethylimidazole (hereinafter sometimes referred to as DMI) (1 weight part per 100 parts by weight of polyamic acid) was added. part, 0.03 mol with respect to 1 mol of the amide group of a polyamic acid), and the polyamic-acid solution was prepared.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com