Catalyst for synthesizing p-tolualdehyde
A technology of p-toluene and catalyst, applied in physical/chemical process catalysts, organic compounds/hydrides/coordination complex catalysts, organic chemistry, etc., can solve the low yield of toluene conversion rate p-toluene And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
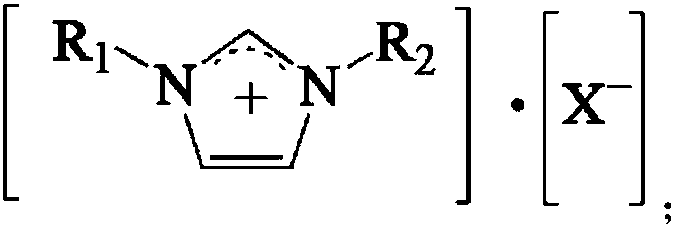
[0047] Add 1-butyl-3-methylimidazolium hexafluoroantimonate (0.5mol, 187g) and Sc(CF 3 SO 3 ) 3 (0.5mol, 246g), the air in the kettle is first replaced with N 2 Replaced 3 times, and then replaced 3 times with CO gas; stirred at 500rpm for 1h; added 110g of toluene; replaced the air in the kettle with CO gas 3 times; Product mixture of methylbenzaldehyde.
[0048] For ease of comparison and illustration, the catalyst formula is listed in Table 1, and the conversion rate of toluene and the yield of p-tolualdehyde are listed in Table 2.
Embodiment 2
[0050] Add 1-butyl-3-methylimidazolium hexafluorophosphate (0.5mol, 142g) and Sc(CF 3 SO 3 ) 3 (0.5mol, 246g), the air in the kettle is first replaced with N 2 Replaced 3 times, then replaced 3 times with CO gas; stirred at 500rpm for 1h; added 110g of toluene; replaced the air in the kettle with CO gas; Product mixture of benzaldehyde.
[0051] For ease of comparison and illustration, the catalyst formula is listed in Table 1, and the conversion rate of toluene and the yield of p-tolualdehyde are listed in Table 2.
Embodiment 3
[0053] Add 1-butyl-3-methylimidazolium hexafluoroantimonate (0.5mol, 187g) and Ce(CF 3 SO 3 ) 3 (0.5mol, 299g); the air in the kettle is firstly used with N 2 Replaced 3 times, and then replaced 3 times with CO gas; stirred at 500rpm for 1h; added 110g of toluene; replaced the air in the kettle with CO gas 3 times; Product mixture of methylbenzaldehyde.
[0054] For ease of comparison and illustration, the catalyst formula is listed in Table 1, and the conversion rate of toluene and the yield of p-tolualdehyde are listed in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com