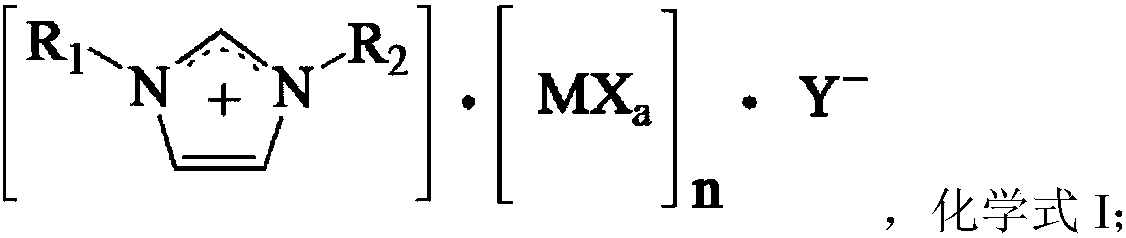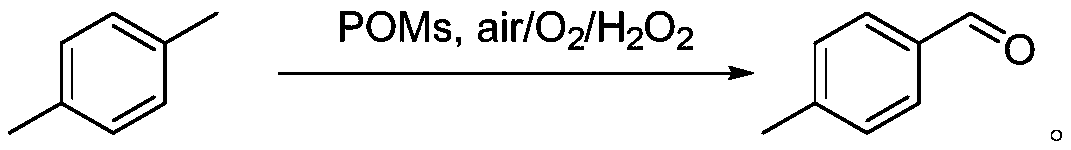Patents
Literature
30 results about "P-tolualdehyde" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of methyl 4-methylcinnamate
InactiveCN102627559AIncrease exposureHigh yieldOrganic compound preparationCarboxylic acid esters preparationDistillationOrganic layer
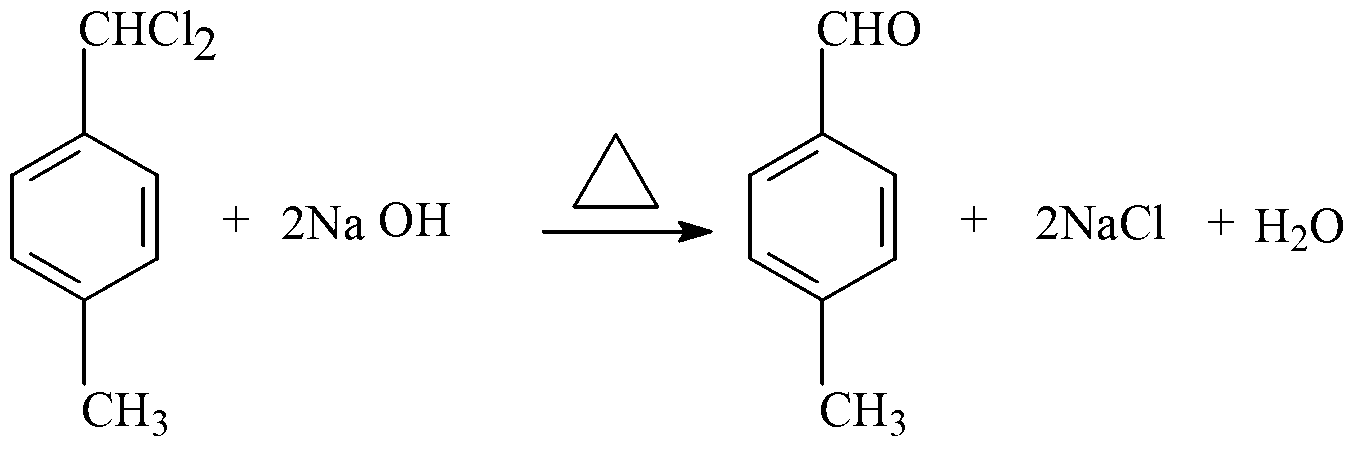
The invention provides a preparation method of methyl 4-methylcinnamate. The preparation method comprises the following steps: stir-mixing glycol dimethyl ether which is used as a solvent, lithium diisopropylamide which is used as a catalyst, and methyl acetate, and slowly adding p-tolualdehyde in a dropwise manner under stirring at below 5DEG C; reacting for 3-6h at 0-10DEG C after ending the dropwise addition, and slowly adding sulfuric acid with the mass percent of 10% in a dropwise manner to regulate the pH of a reaction solution between 6.8-7.2; and separating the resulting organic layer, carrying out reduced pressure distillation to recover the solvent and excess p-tolualdehyde and obtain a white solid, washing the white solid with water, drying to obtain crude methyl 4-methylcinnamate, and recrystallizing the crude methyl 4-methylcinnamate to obtain methyl 4-methylcinnamate. The preparation method which has the advantages of low costs of raw materials, mild reaction condition, preparation of methyl 4-methylcinnamate through a one-step reaction, simplicity, easy operation, high yield, stable and reliable product quality, and no pollution to environment is a green synthetic method.
Owner:湖北远成赛创科技有限公司
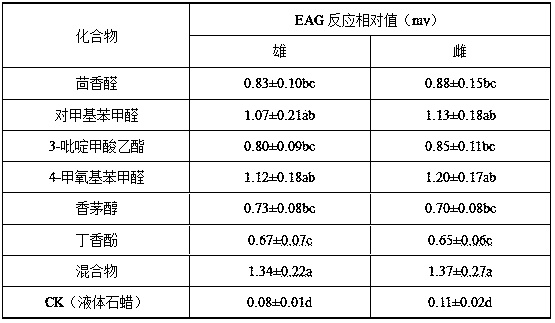
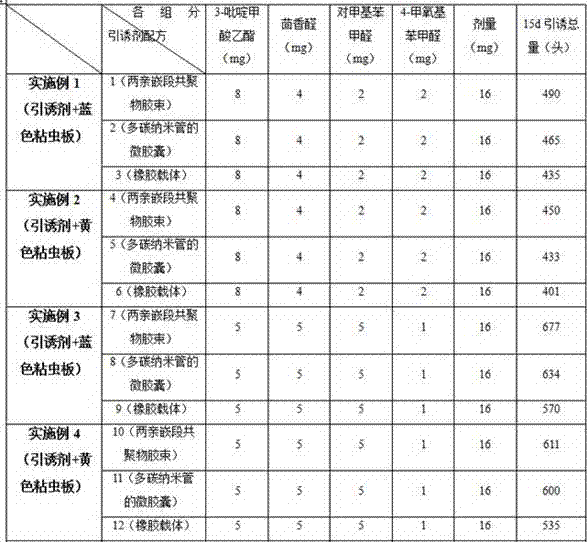
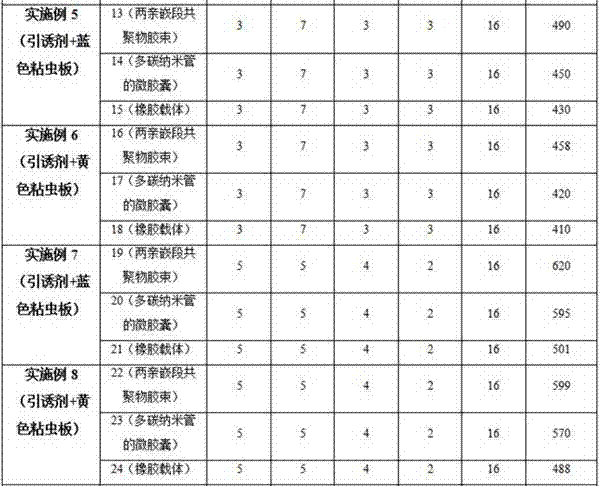
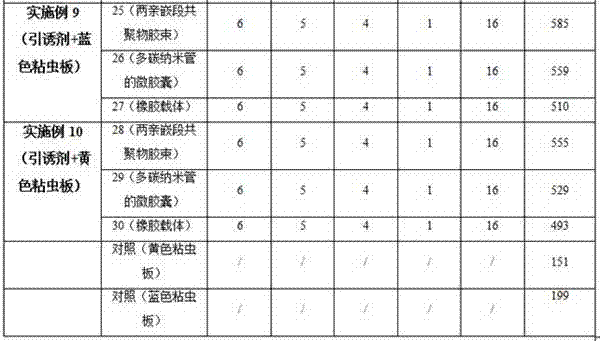
Thrip attractant and application thereof
The invention discloses a thrip attractant and an application thereof. The thrip attractant is prepared from ethyl 3-pyridinecarboxylate, anisaldehyde, p-tolualdehyde and p-methoxybenzaldehyde in a mass ratio being (1-20):(1-20):(1-20):(1-10) through mixing; the substances are dissolved in n-hexane and slowly released in a carrier, lure is prepared, and the lure carrier of the thrip attractant is suspended on a blue or yellow pest sticking board. Control tests indicate that under the application of the thrip attractant, the control effect is similar to that of independently applied pesticides, and the control effect can be improved 3-4.5 times compared with that of the independently applied pest sticking board. When applied to control for thrips, the thrip attractant has the characteristics of being stable in product performance, long in persistent period, efficient and environment-friendly, luring numerous varieties and the like, the use quantity of chemical pesticides is substantially reduced, the damage rate of vegetables, fruits or flowers is also effectively controlled, sustainable development of green products is promoted, and a new thought is provided for the integrated control technique of thrip pests.
Owner:INST OF PLANT PROTECTION FAAS
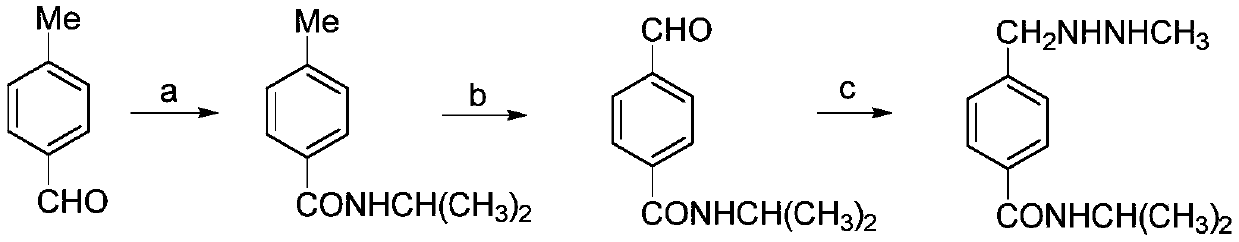
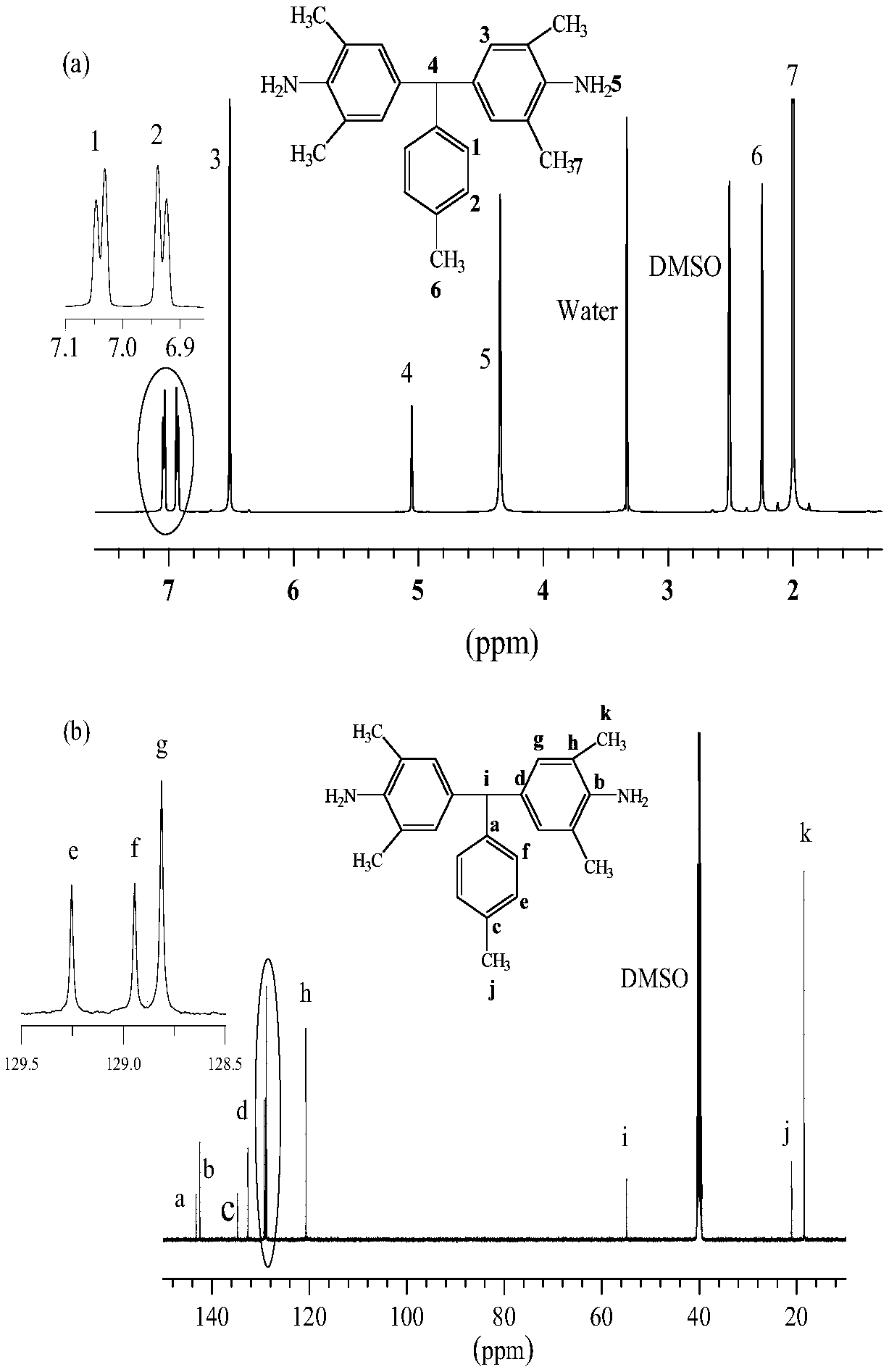
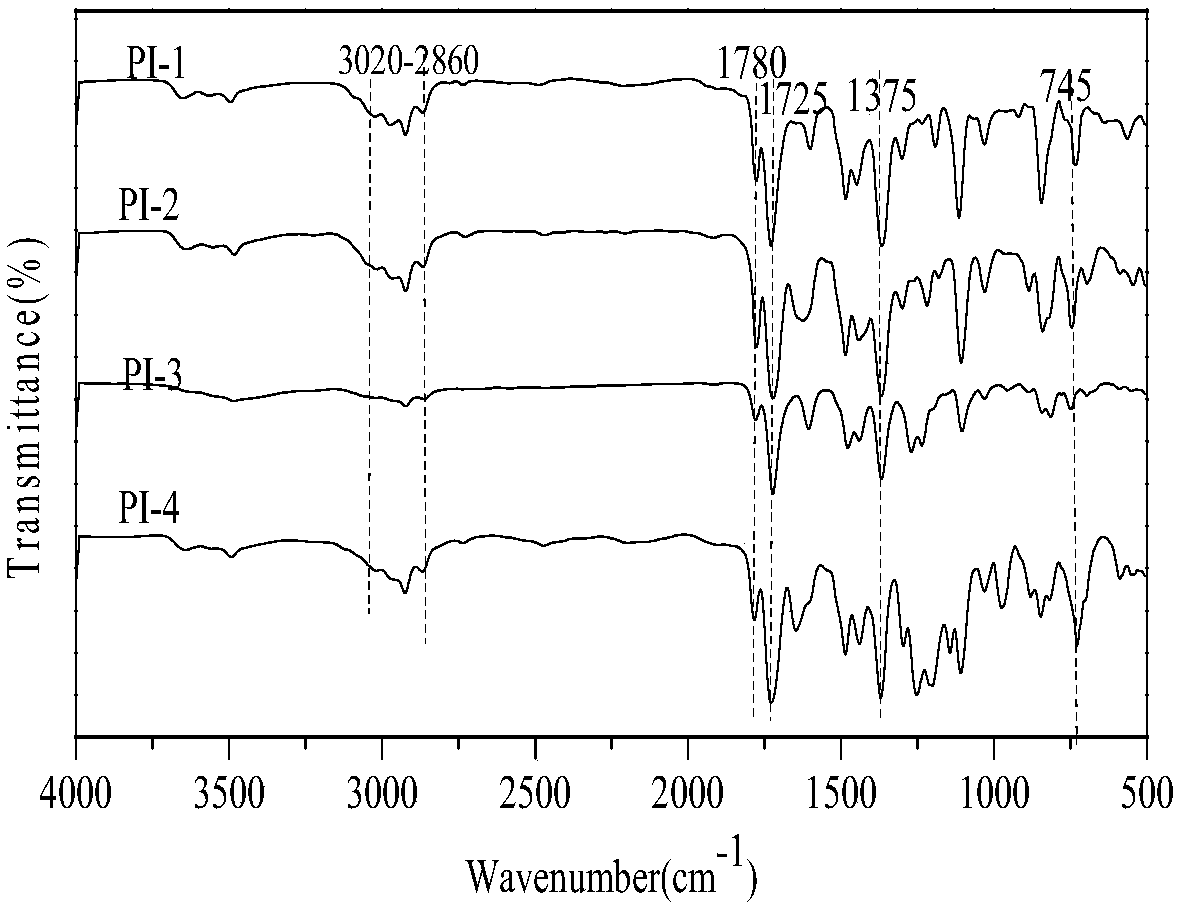
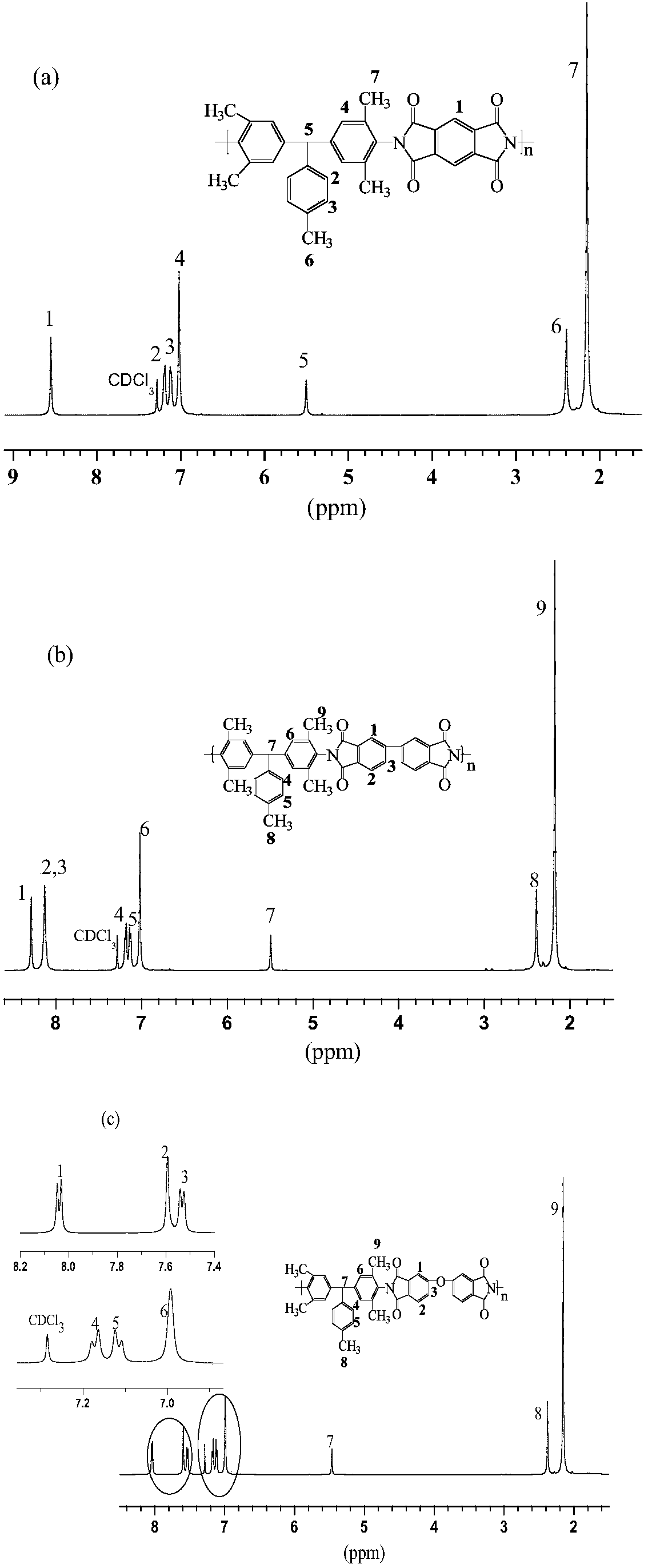
Aromatic diamine and polyimide containing tolyl and non-coplanar structure and their preparation methods
InactiveCN108530304APrevent Schiff base reactionSlow reaction rateOrganic compound preparationAmino compound preparationSolubilityDecomposition
The invention discloses aromatic diamine and polyimide containing tolyl and non-coplanar structure and their preparation methods. A general formula of the aromatic diamine is shown in the description.The preparation method of the aromatic diamine includes: allowing 2,6-dimethylaniline and p-tolualdehyde to react at the temperature of 80-110 DEG C under the protection of excessive hydrochloric acid in the nitrogen atmosphere, with water serving as a solvent, to obtain crude aromatic diamine; performing normal butanol recrystallization to obtain polymeric aromatic diamine, up to 90% in yield; subjecting the polymeric aromatic diamine and different dianhydrides to one-step high temperature polycondensation to obtain polyimide. The glass transition temperature herein is up to 376 DEG C; 5% thermogravimetric decomposition temperature is up to 517 DEG C, with very high thermal stability shown; diamine monomer substitutive methyl group is a nonpolar group and is of symmetric substitution such that the synthesized nonpolar polymeric polyimide has dielectric constant down to 2.47 under 1 KHz, with excellent solubility; the forming technique temperature of polyimide is lowered.
Owner:WUHAN UNIV OF TECH
Oxaphosphaphenanthrene fire retardant containing cyclotriphosphazene and cup [4] aromatic ring structures as well as preparation method and application of oxaphosphaphenanthrene fire retardant
ActiveCN104262682AEasy to operateAdvanced technologyGroup 5/15 element organic compoundsEpoxyBenzaldehyde
The invention provides an oxaphosphaphenanthrene fire retardant containing cyclotriphosphazene and cup [4] aromatic ring structures as well as a preparation method and application of the oxaphosphaphenanthrene fire retardant. The preparation method comprises the steps that hexa-p-hydroxy phenoxy cyclotripolyphosphazene and DOPO react in an organic solvent to obtain an oxaphosphaphenanthrene intermediate I containing a cyclotriphosphazene structure; p-toluenesulfonate ethoxyl p-tolualdehyde and p-tertiary butyl cup [4] aromatic hydrocarbon react under the action of acetonitrile-K2CO3 to obtain an intermediate II; the intermediate I, the intermediate II and hydrazine hydrate react in the organic solvent to obtain the oxaphosphaphenanthrene fire retardant containing the cyclotriphosphazene and cup [4] aromatic ring structures. The oxaphosphaphenanthrene fire retardant containing the cyclotriphosphazene and cup [4] aromatic ring structures is white in appearance, melting point is 212-214 DEG C, thermal stability is good, flame retardant rate is high, and purity is 99.1%; the used raw materials are available, a technology is advanced, and industrial production can be easily realized. The oxaphosphaphenanthrene fire retardant containing the cyclotriphosphazene and cup [4] aromatic ring structures can be taken as a reactive type fire retardant and can also be taken as an additive flame retardant when being used after being grafted to thermosetting resins of epoxy resin, polyurethane and the like, so as to meet the halogen-free fire retardant requirement of engineering plastics, with high heat resistance requirement on fire retardant, such as ABS, nylon and the like.
Owner:湖北君邦新材料科技有限公司
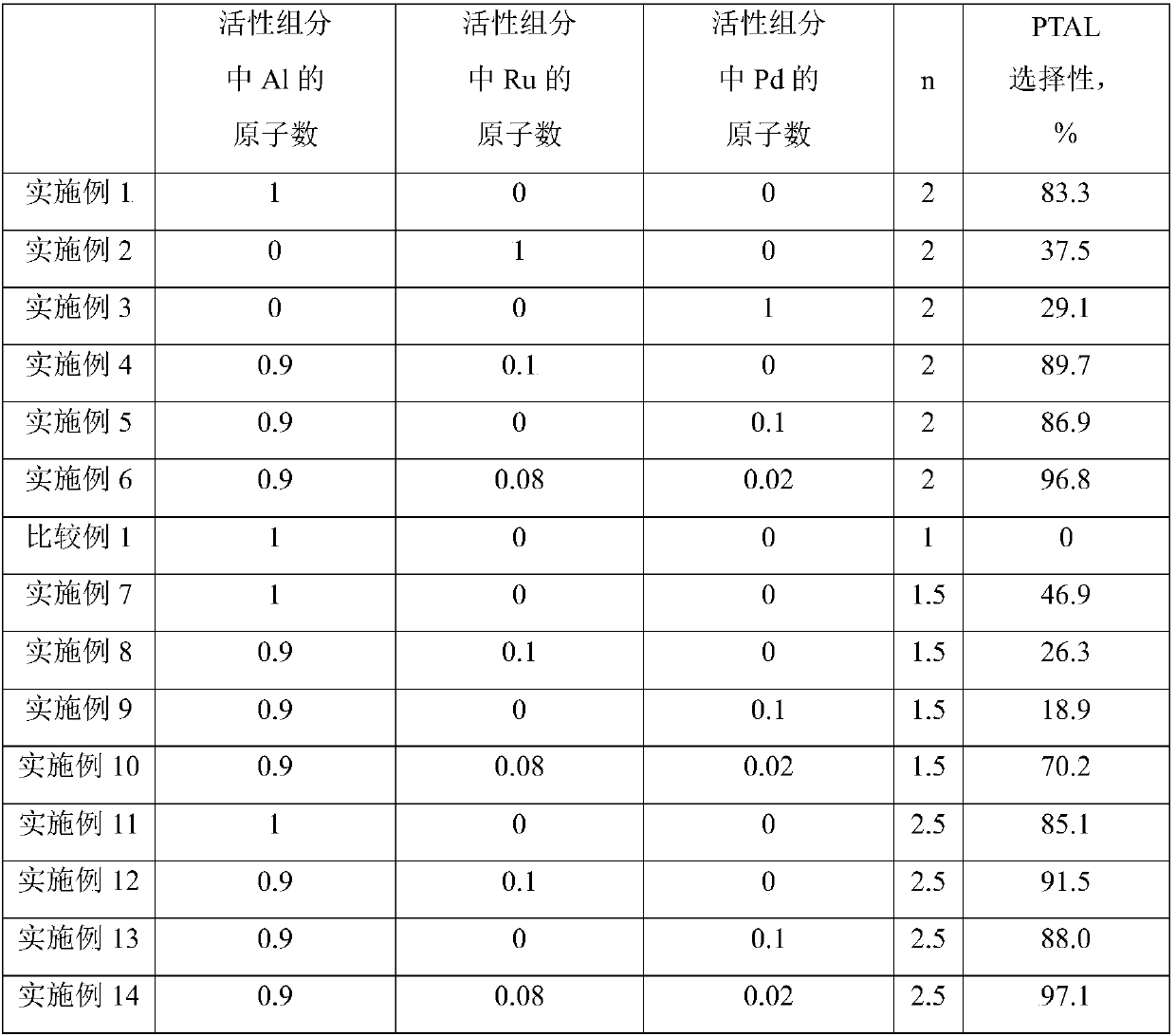
Catalyst used for synthesis of p-tolualdehyde
ActiveCN107866283AHigh selectivityBeneficial technical effectOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionBromineActive component
The invention relates to a catalyst used for synthesis of p-tolualdehyde. The problem that p-tolualdehyde is low in selectivity in the prior art is mainly solved. The catalyst comprises an active component which is a compound shown in the following formula (in the description). In the formula, R1 and R2 are independently selected from C1-C10 alkyl, M is selected from at least one of Al or palladium-series metal, X and Y are independently selected from chlorine or bromine, a is the absolute value of the valence of M, and N is more than 1 and less than 5. The technical problem is solved. The catalyst can be used for industrial production of p-tolualdehyde.
Owner:CHINA PETROLEUM & CHEM CORP +1
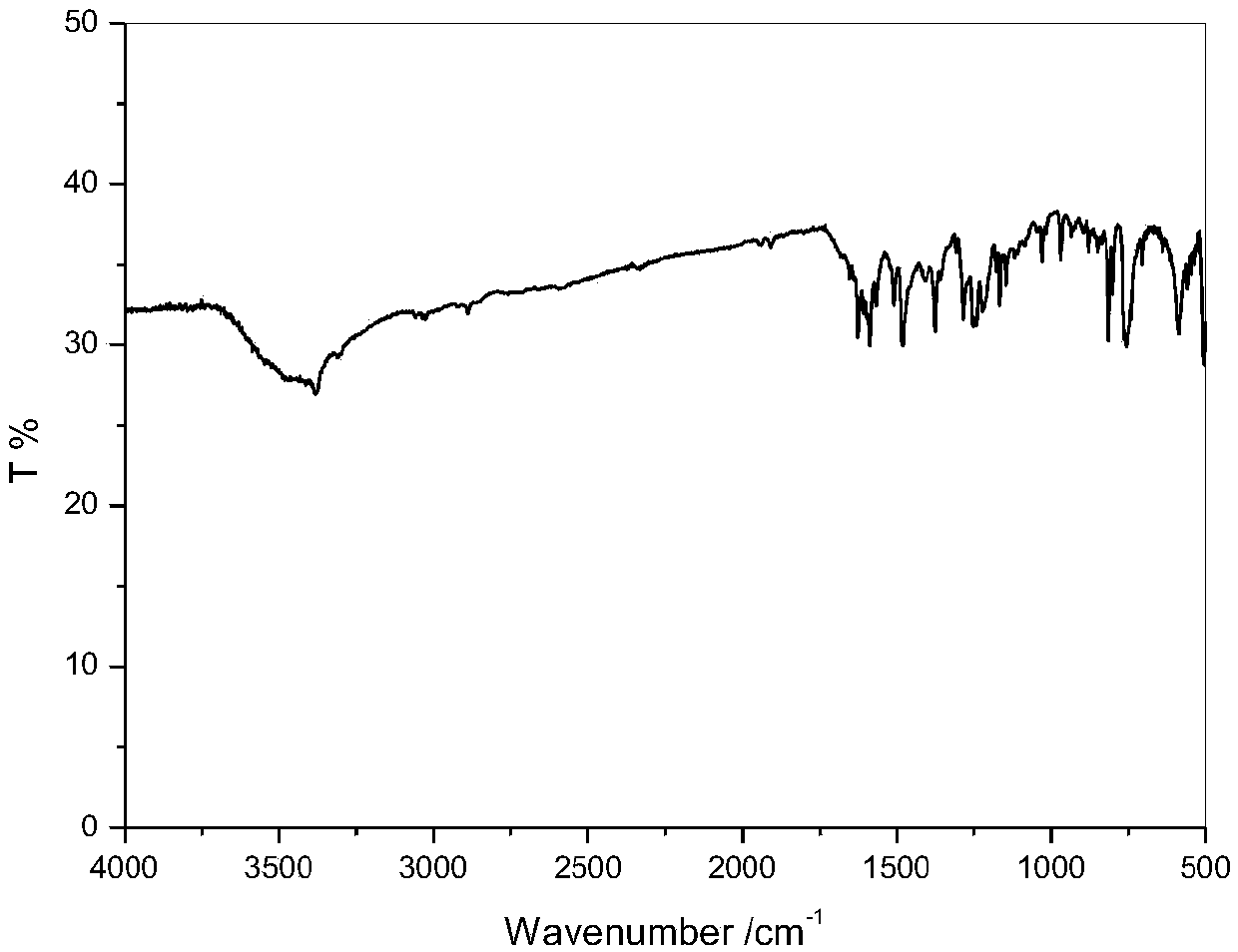
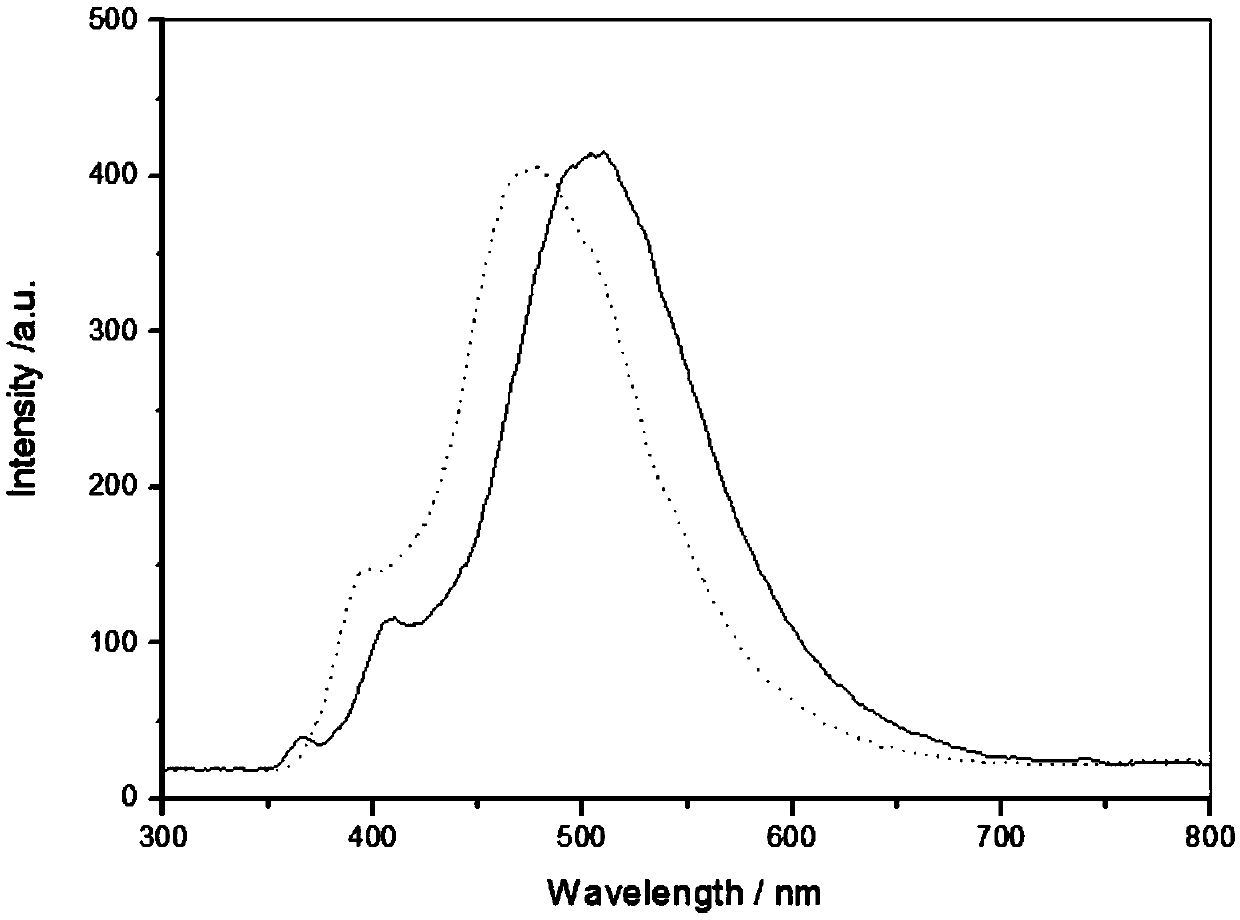
Method for preparing p-tolualdehyde by efficiently catalyzing p-xylene by polyoxometallate
InactiveCN110078601AHigh yieldEasy to makeOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by oxidationOrganic solventPolyoxometalate I
The invention relates to a method for preparing p-tolualdehyde by efficiently catalyzing p-xylene by polyoxometallate. Polyoxometallate as a catalyst is placed in a reactor, an organic solvent and p-xylene are sequentially added to the reactor, finally, an oxidizing agent is added, a stirring reaction is performed at 50-80 DEG C for 12-48 h, and p-tolualdehyde is separated. Compared with the priorart, the method is simple to operate and mild in condition and is a p-tolualdehyde preparation method with atom economy and environmental friendliness, the conversion rate of p-xylene and selectivityof p-tolualdehyde are high, and the catalyst has the characteristics of being green, efficient, easy to recycle and the like and has promotion and utilization values.
Owner:SHANGHAI INST OF TECH
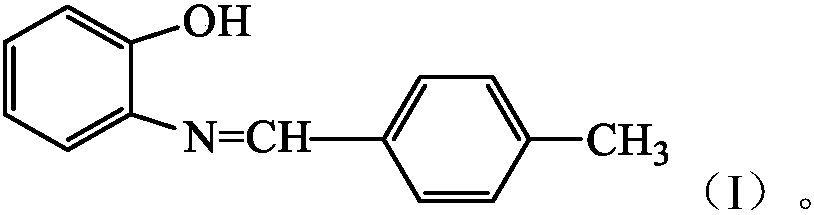
Schiff base derivative and preparation method thereof
InactiveCN107673991AHigh yieldEfficient responseLuminescent compositionsImino compound preparationEnvironmental resistanceOrganic solvent
The invention discloses a Schiff base derivative and a preparation method thereof. A structural formula of the Schiff base derivative is as shown in the specification. The preparation method includes:subjecting ortho-aminophenol and p-tolualdehyde in a molar ratio of 1:1-2 to reaction in a hydrothermal reaction kettle for 180-360min at a reaction temperature of 90-170 DEG C, wherein the water filling degree of the hydrothermal reaction kettle is 30-70%; after reaction is finished, subjecting reaction products to filtering, drying and recrystallizing to obtain the Schiff base derivative. By substitution of a conventional organic solvent with water as a reaction medium without use of any catalysts, nontoxicity, harmlessness and environmental friendliness are realized. In addition, by reaction material selection and reaction condition optimization, yield of the prepared Schiff base derivative is greatly increased and maximally reaches 68.8% which is evidently higher than the yield of theSchiff base derivative prepared according to an existing similar method.
Owner:CHONGQING UNIV OF TECH
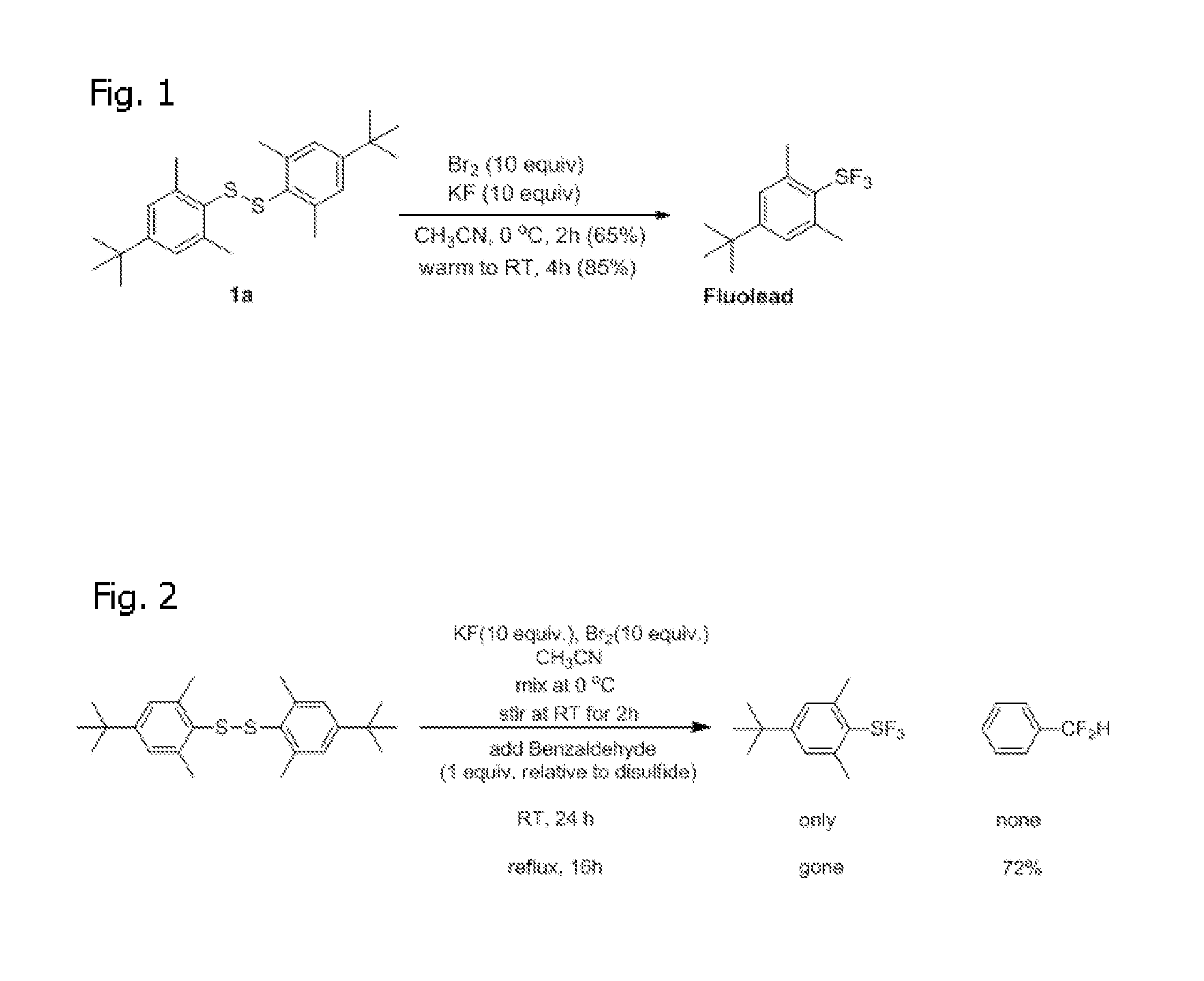
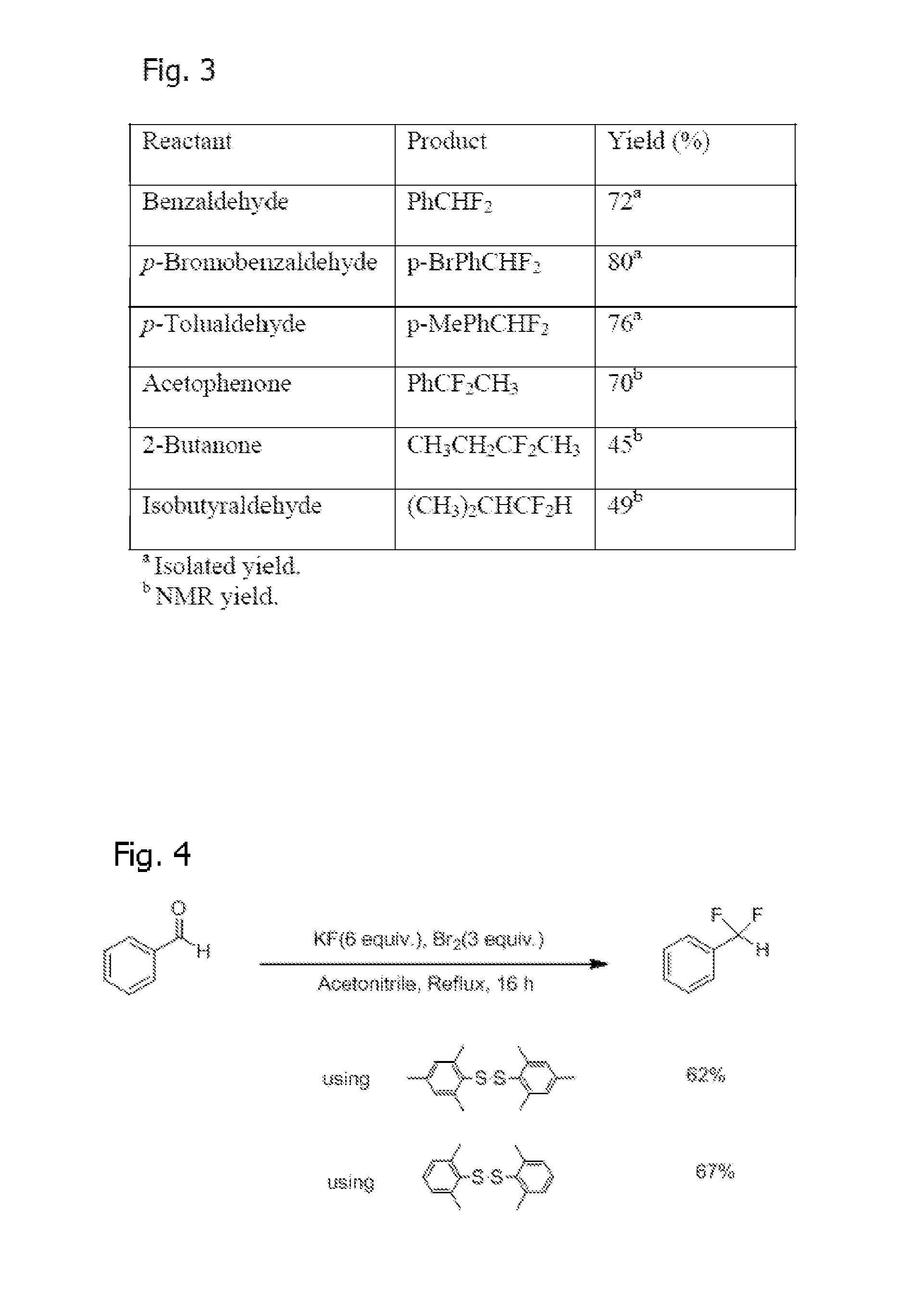
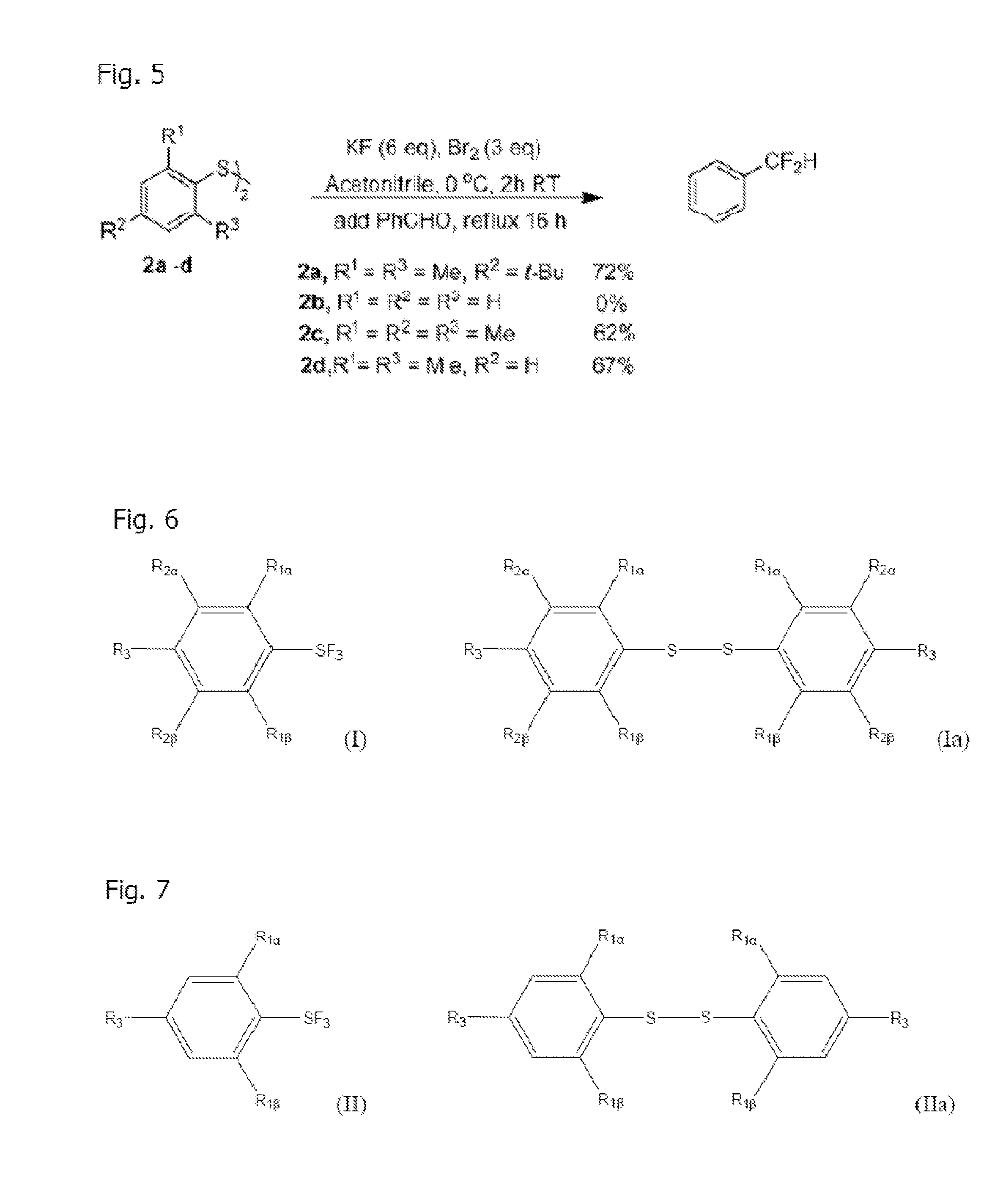
Method of Synthesis of Arylsulfur Trifluorides and Use as in situ Deoxofluorination Reagent
InactiveUS20120083627A1Reduce reactivityReduce susceptibilityOrganic compound preparationHalogenated hydrocarbon preparationBenzaldehydeTrifluoride
The invention is a method of synthesizing Arylsulfur Trifluorides, such as Fluolead, by reacting BR2 and KF (or suitable alkali metal fluoride) in acetonitrile (or other suitable solvent). The invention also comprises using the Fluolead (or its substitutes), thus prepared, in situ as deoxofluorination reagents with a suitable aldehyde, ketone, or alcohol such as one selected from the group consisting of benzaldehyde Benzaldehyde, p-Bromobenzaldehyde, p-Tolualdehyde, Acetophenone, 2-Butanone, or Isobutyraldehyde, wherein the mixture is heated to reflux until completion. The respective products are then isolated after extraction by hexane and destruction of the sulfinyl fluoride co-product.
Owner:FLUOROTECH +1
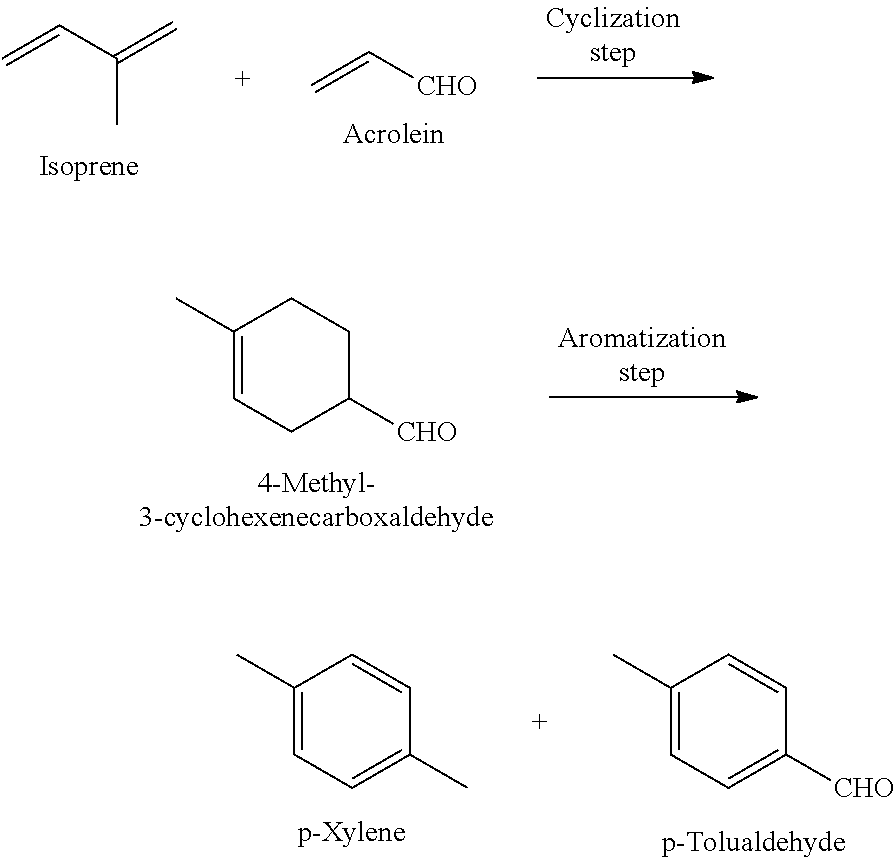
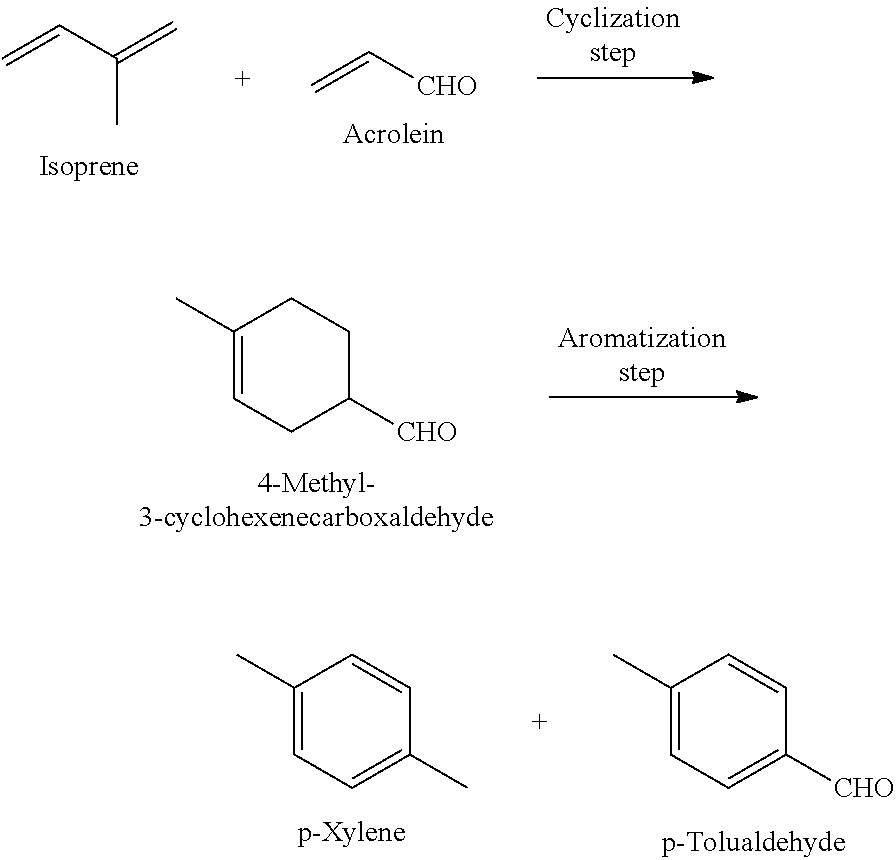
Method for producing p-xylene and/or p-tolualdehyde
InactiveUS20140378710A1High yieldShort processOrganic compound preparationCatalystsGas phaseAromatization
Disclosed is a method for producing p-xylene and / or p-tolualdehyde with high yield through a short process using biomass resource-derived substances as raw materials. The method for producing p-xylene and / or p-tolualdehyde of the present invention comprises: a cyclization step of producing 4-methyl-3-cyclohexenecarboxaldehyde from isoprene and acrolein; and an aromatization step of producing p-xylene and / or p-tolualdehyde from 4-methyl-3-cyclohexenecarboxaldehyde by gas-phase flow reaction using a catalyst(s).
Owner:TORAY IND INC
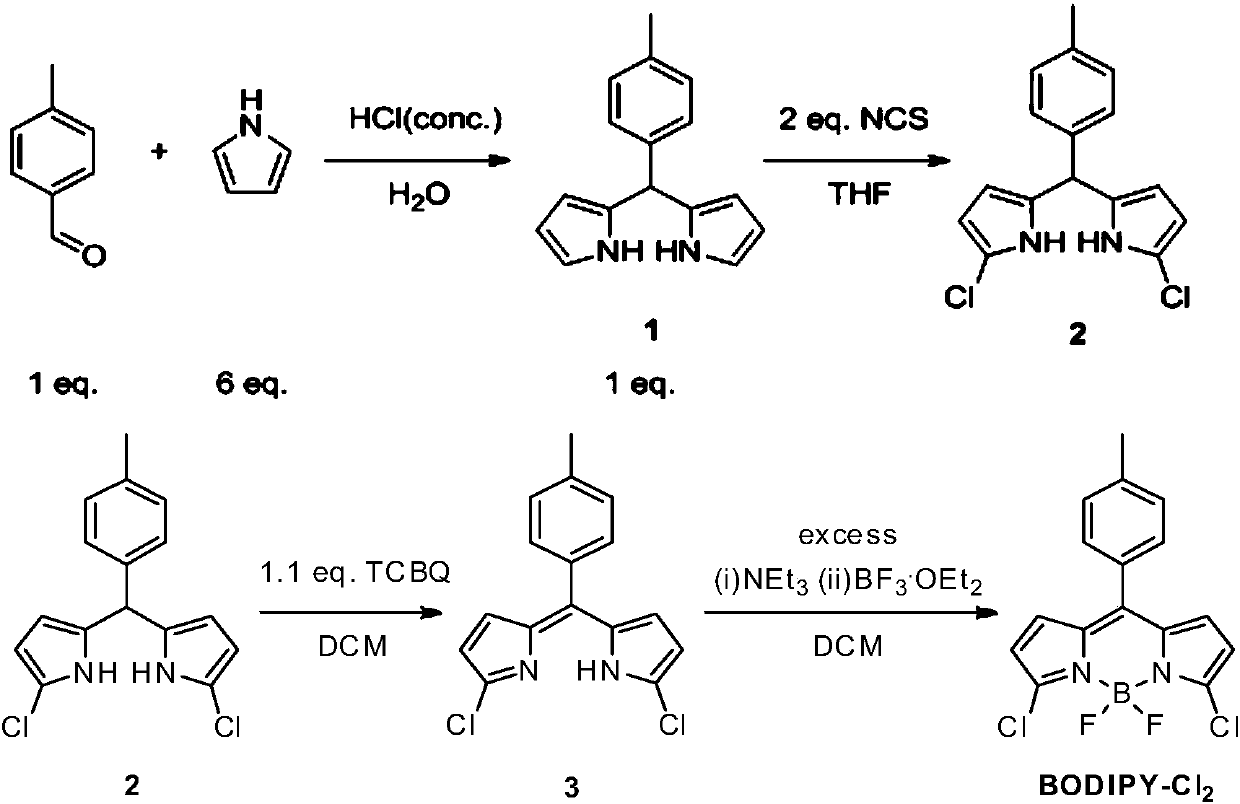
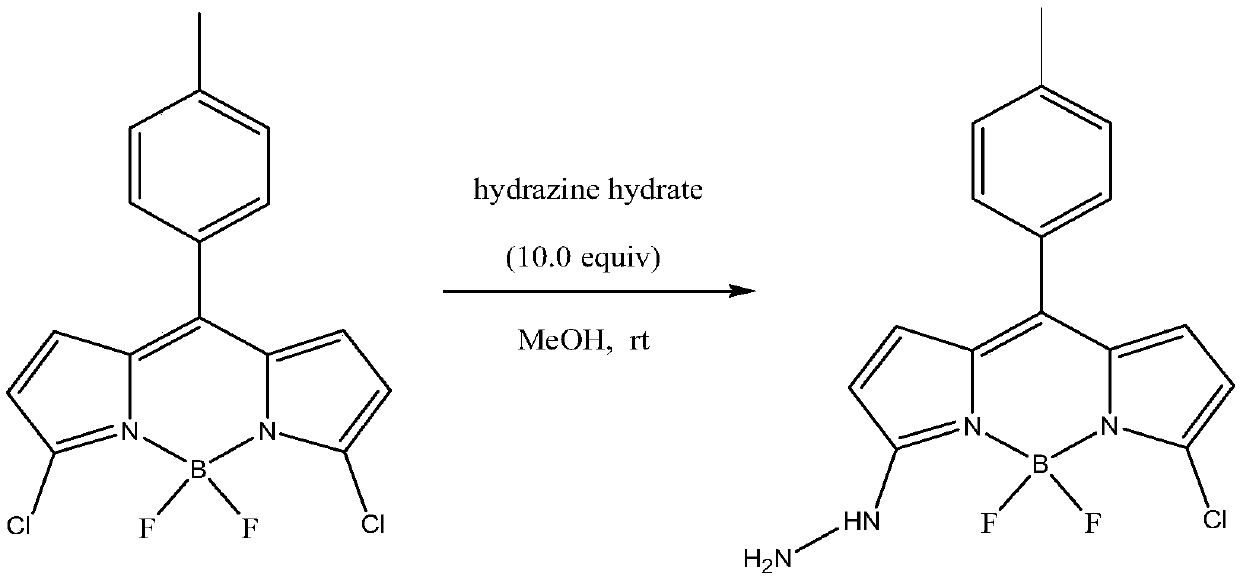
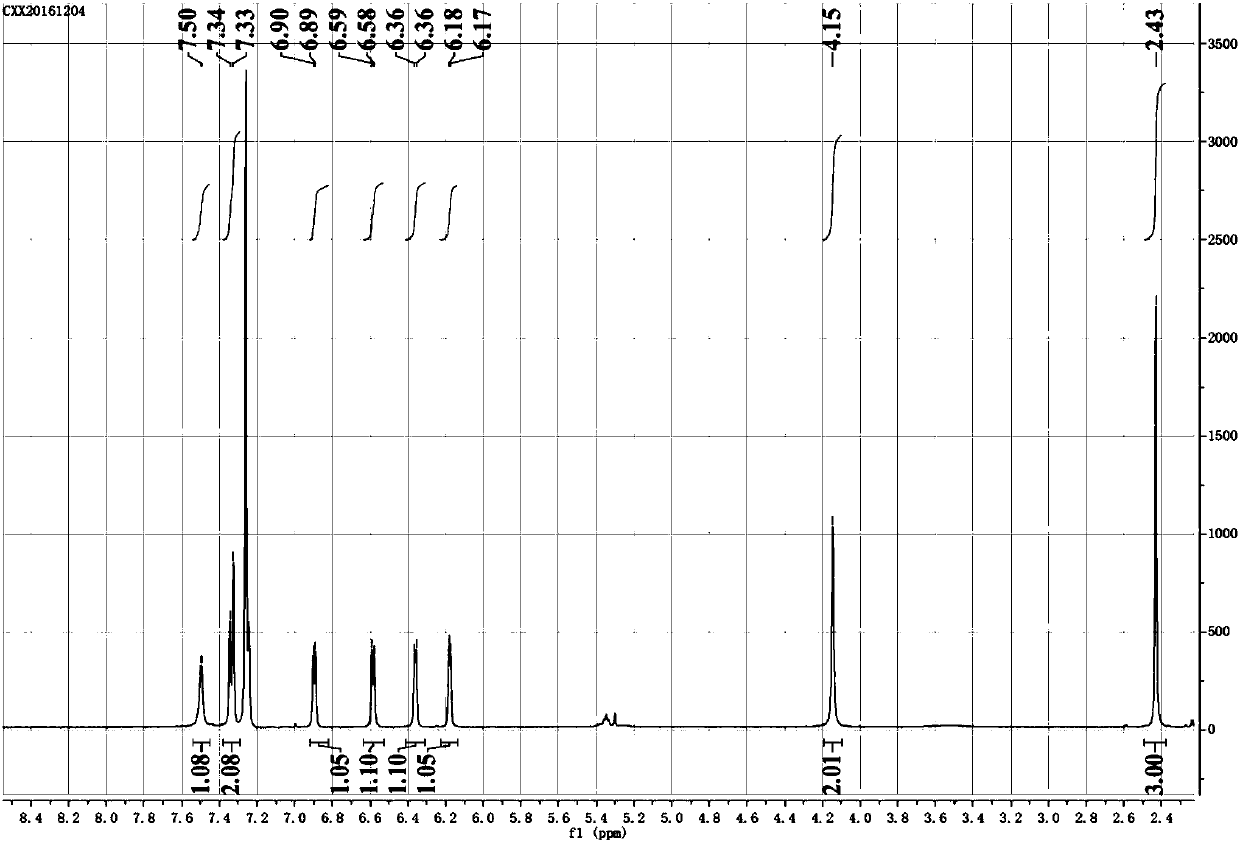
Fluorescent probe molecule for detecting explosive RDX and preparation method and application of fluorescent probe molecule
ActiveCN109748930AReduce manufacturing costThe synthetic route is simpleGroup 3/13 element organic compoundsFluorescence/phosphorescenceResponse sensitivityRoom temperature
The invention relates to a fluorescent probe molecule for detecting explosive RDX and a preparation method and application of the fluorescent probe molecule. The preparation method includes: using pyrrole and p-tolualdehyde as the reactants to prepare a chloro-BODIPY derivative, allowing chloro-BODIPY derivative to have reaction with hydrazine hydrate at room temperature for 12 hours, and performing column chromatography isolation to obtain the hydrazine-substituted fluorescent probe molecule. RDX is detected indirectly by the response of the fluorescent probe molecule to explosive RDX photolysis products. The preparation method is mild in reaction conditions and simple in test conditions. The probe molecule is high in response sensitivity to explosive RDX and good in selectivity and is afeasible attempt for applying fluorescent sensors to explosive detection.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
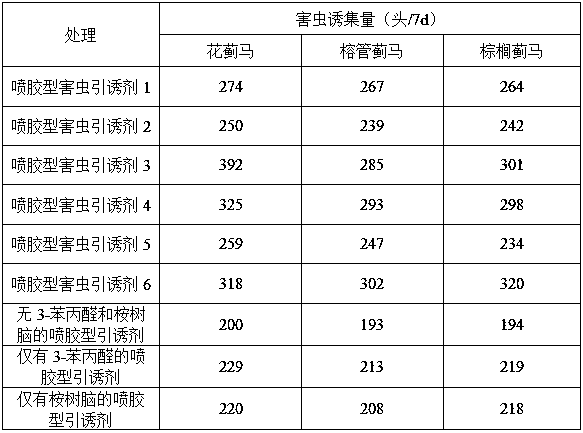
Glue-spraying type pest attractant
InactiveCN108575992AReduce usageImprove performanceBiocidePest attractantsMass ratioEucalyptus gomphocephala
The invention relates to a glue-spraying type pest attractant. The attractant comprises the following components in parts by weight: 90-95 parts of pest sticking glue, 3-8 parts of insect pheromone, 0.1-3 parts of 3-phenylpropionaldehyde and 0.1-3 parts of eucalyptol. The insect pheromone is prepared from anisaldehyde, p-tolualdehyde, ethyl 3- piperidinecarboxylate, 4-anisaldehyde, citronellol andeugenol according to the mass ratio of (1-15):(1-15):(1-15):(1-5):(1-5):(1-5). According to the invention, the compounded 3-phenylpropionaldehyde and the eucalyptol are used as plant-source synergists and can improve the attracting effect of the insect pheromone by 20%-35%. Meanwhile, the oily glue is utilized as a release agent and is sprayed on appliance surfaces, and when a diluent is volatilized, the high binding character of the colloid is utilized for high efficiently attracting and killing pests. The pest attractant provided by the invention meets the urgent requirements of glue-spraying type pest attractants with no public nuisance, high efficient attraction, good stickiness, high strength, good stability, simple and convenient operation and low cost on the market, and has a widemarket prospect.
Owner:INST OF PLANT PROTECTION FAAS
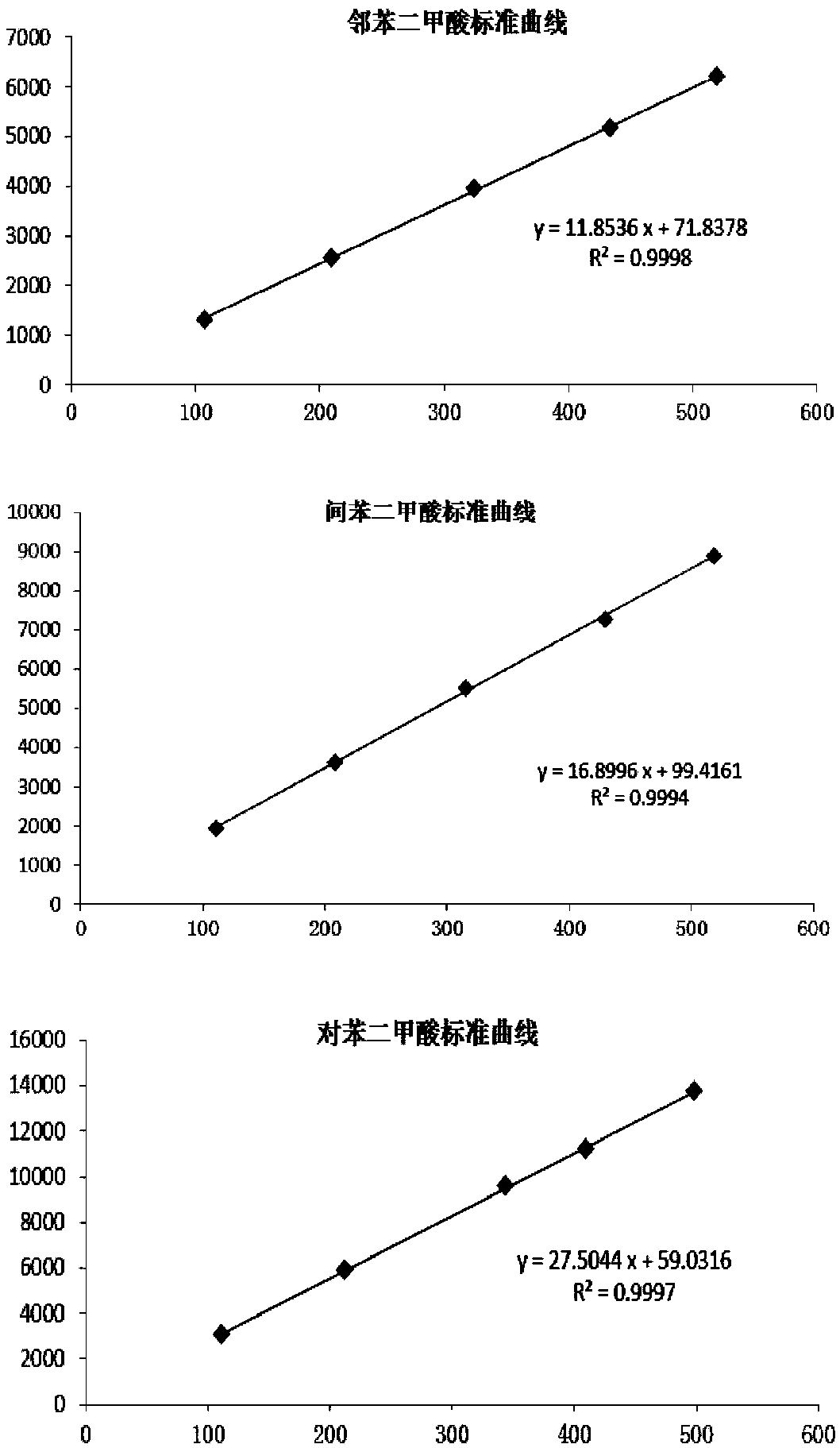
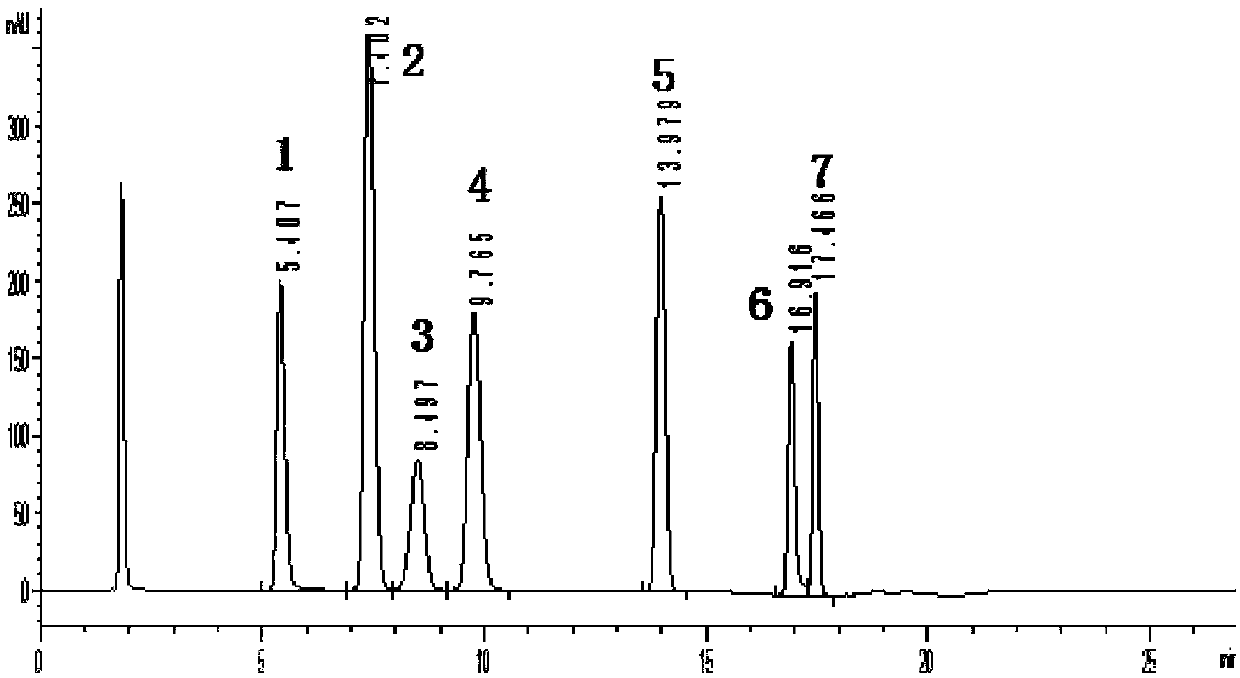
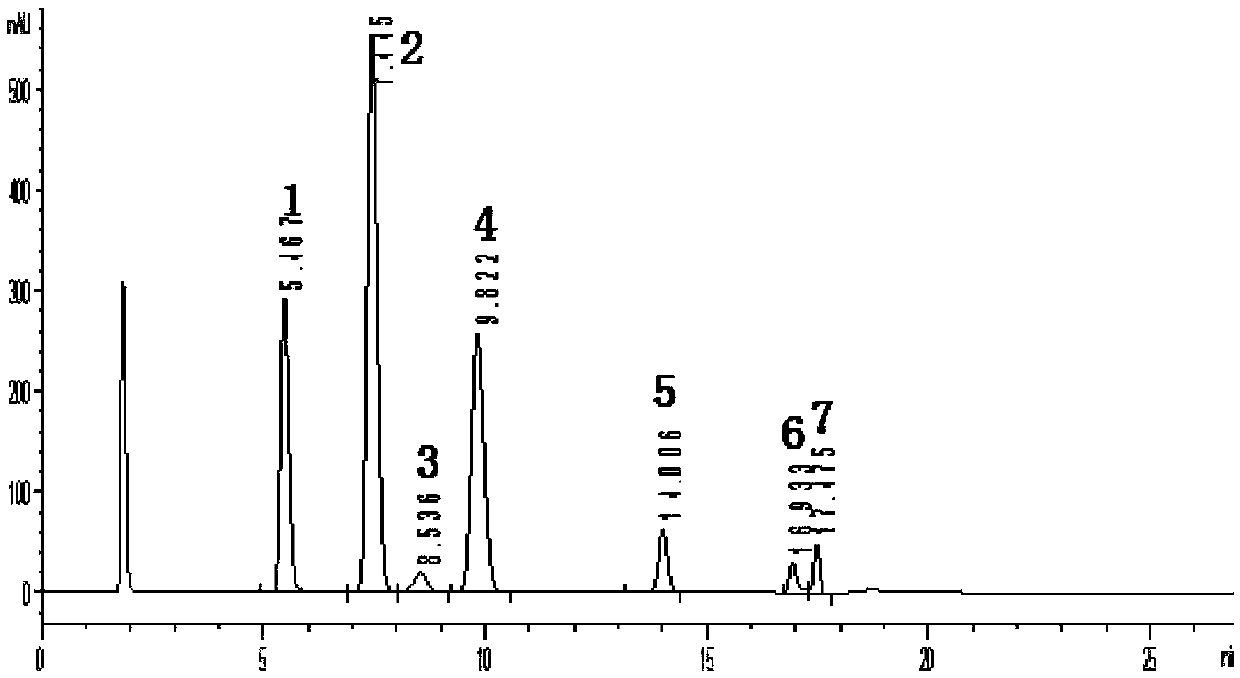
Method for measuring content of dimethylbenzene oxidation products by using HPLC external standard method
InactiveCN107907615AEfficient separationShort analysis timeComponent separationBenzoic acidUv vis absorbance
The invention discloses a method for measuring content of dimethylbenzene oxidation products by using an HPLC external standard method. According to the method, a high performance reversed-phase liquid chromatograph instrument is adopted, a C18 silica gel chromatographic column is taken as a separation column, an ultraviolet visible absorption detector is adopted, methanol and an acid aqueous solution are taken as mobile phases, phthalate, isophthalic acid and terephthalic acid are taken as standard samples, a concentration-peak area standard curve of a target component is drawn by using the external standard method so as to obtain a linear regression equation, and contents of the phthalate, isophthalic acid and terephthalic acid are calculated by using the regression equation. Through adoption of the method, three main products with similar polarity and four byproducts including 3-formylbenzoic acid, benzoic acid, paratoluic acid and p-tolualdehyde can be separated effectively, and the method takes short time in analysis, is easy and feasible, and is convenient and reliable.
Owner:CHAMBROAD CHEM IND RES INST CO LTD
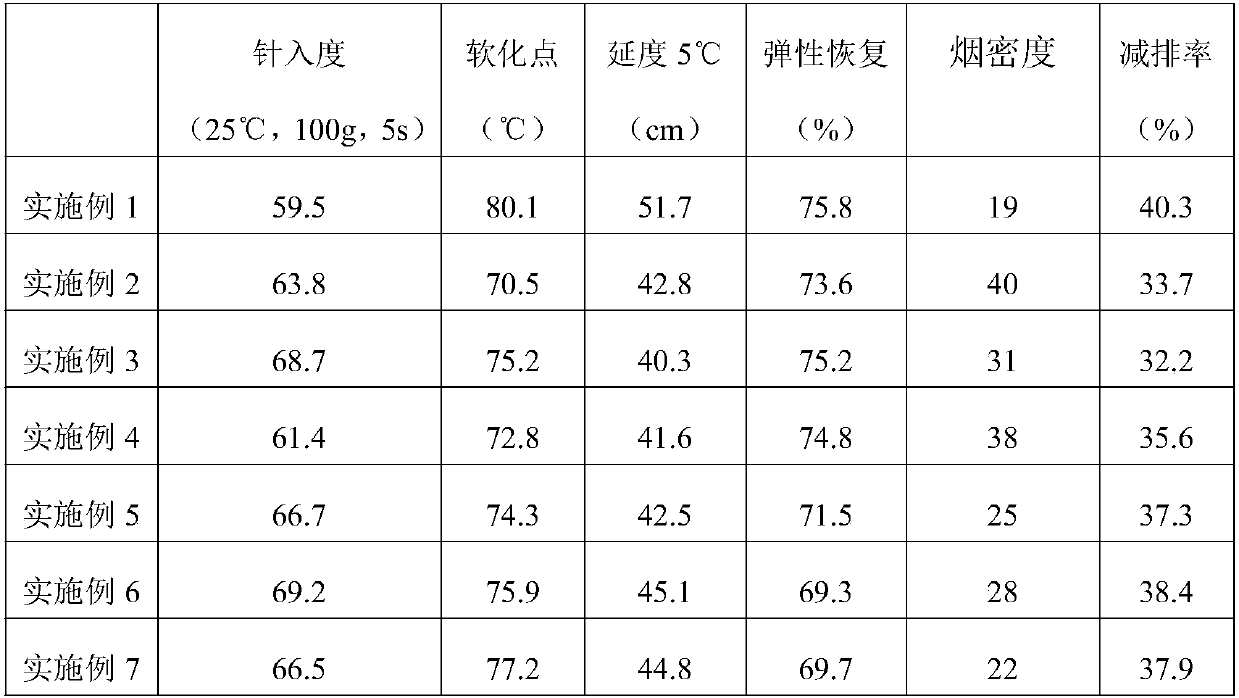
Modified road asphalt
InactiveCN108034267AHigh softening pointReduce penetrationBuilding insulationsNeedle penetrationCrack resistance
Owner:杭州睿琦化工科技有限公司
Road asphalt
The invention discloses road asphalt. The road asphalt is prepared from the following components by weight percentage: 80-100 parts of asphalt, 3-15 parts of styrene butadiene rubber, 2-5 parts of neoprene, 2-5 parts of isoprene rubber, 2-6 parts of polystyrene, 0.3-3 parts of N-isopropyl-N-phenyl-p-phenylenediamine, 0.5-5 parts of dimethyl phthalate, 0.1-1 part of p-tolualdehyde, 3-10 parts of mica powder, 1-7 parts of sodium alkyl benzene sulfonate, 3-8 parts of polyvinyl alcohol and 1-3 parts of caustic soda. The road asphalt has a good interlayer bonding degree, the high-temperature stability performance of the asphalt can be effectively improved, and meanwhile, the brittleness temperature is decreased so that the low-temperature anti-cracking performance of the asphalt can be improved; the asphalt is high in viscosity, resistant to high temperature and good in waterproof property, and has high ability of resisting high-temperature and rainy weather, and thus, the damage rate of roads is greatly decreased.
Owner:海宁新业新材料科技有限公司
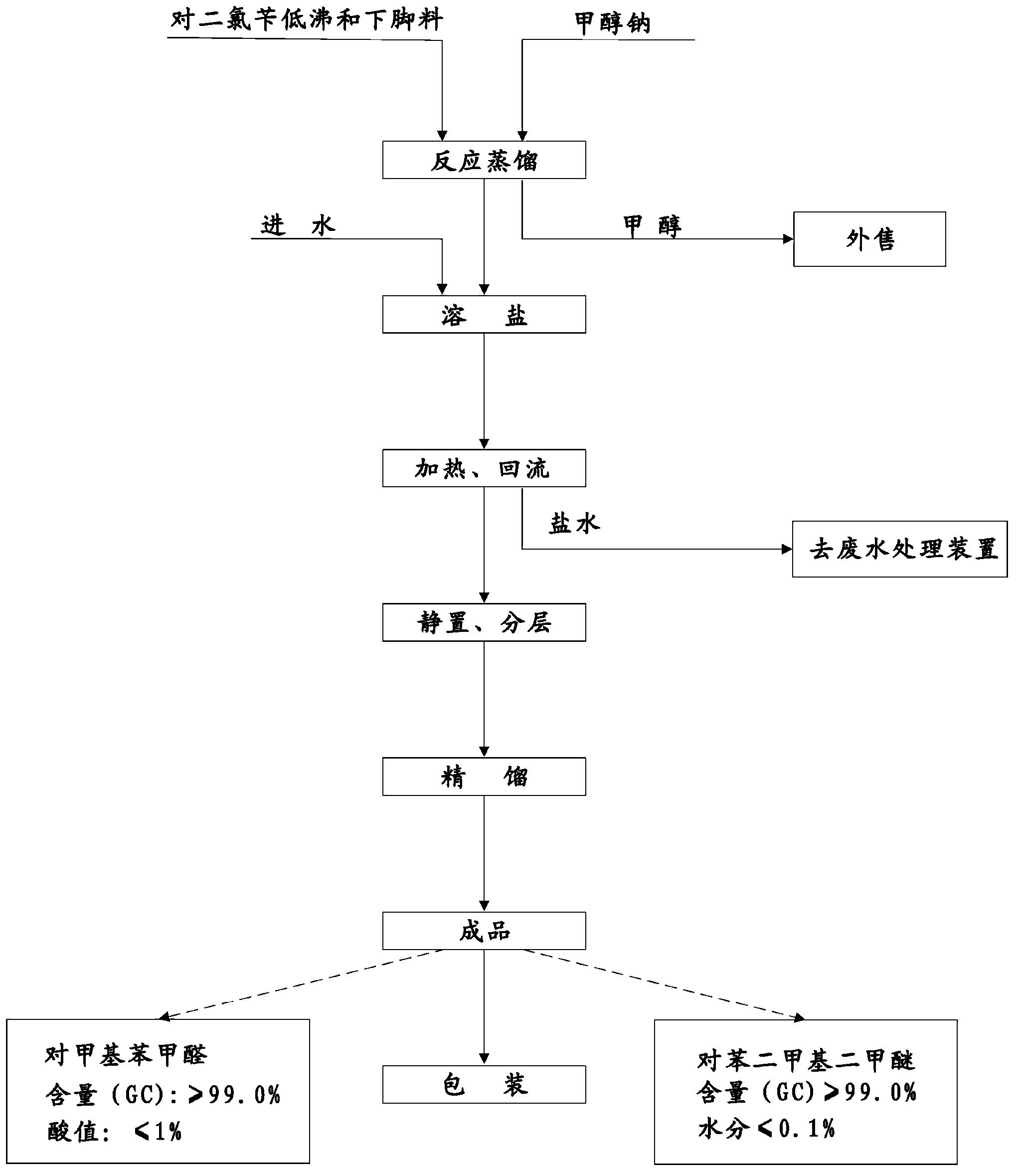
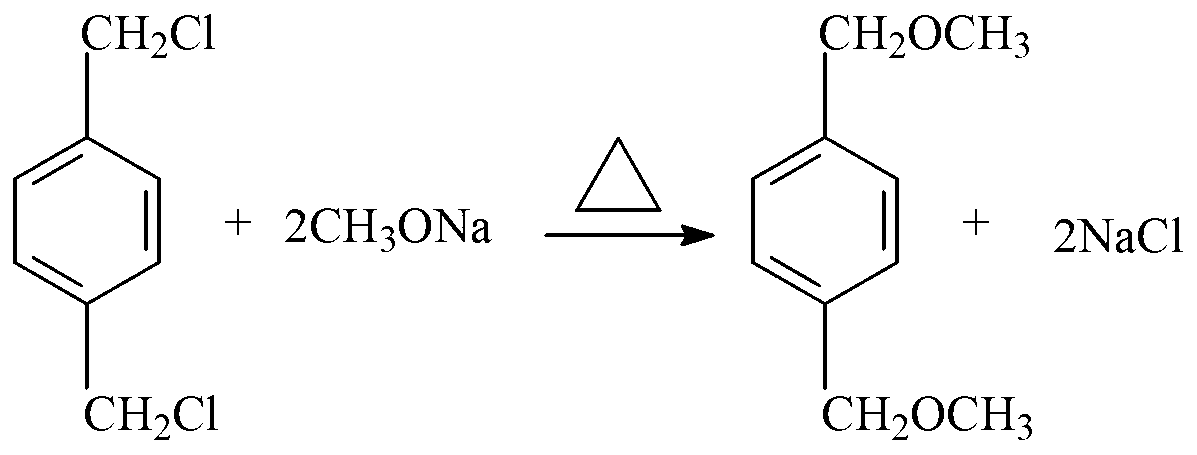
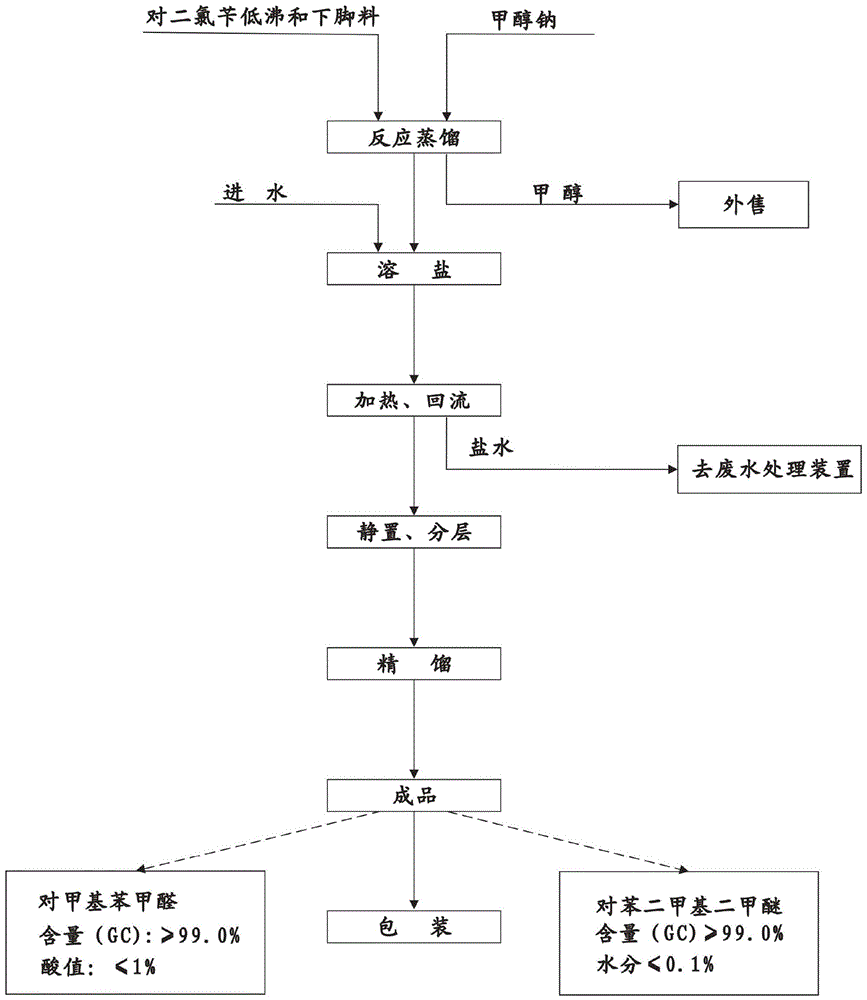
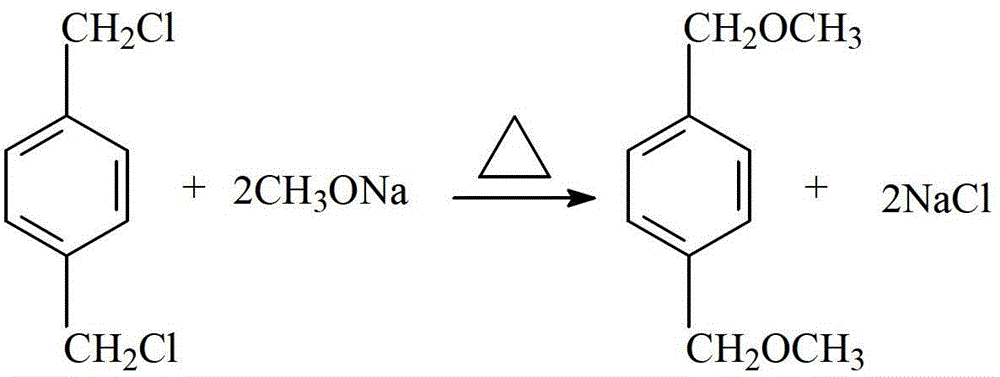
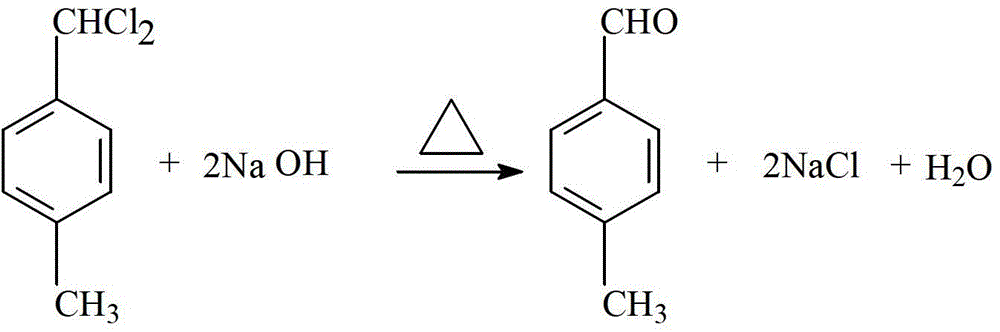
Novel process of 1,4-bis(methoxymethyl)-benzene preparation method
ActiveCN103288604AShorten the production cycleEasy to operateCarbonyl compound preparation by hydrolysisEther preparationXylyleneBenzene
The invention provides a novel process of a 1,4-bis(methoxymethyl)-benzene preparation method. The novel process comprises the following steps of: (1) reaction distillation: heating low-boiling leftovers containing p-xylylene dichloride to reach the temperature above 110 DEG C, pumping into a reaction kettle under vacuum; adding sodium methoxide solution to react and distil at temperature below 80 to 100 DEG C, stopping reaction to obtain a crude product while the content of the p-xylylene dichloride residual in a reactant accounts for 0.05 to 0.5% (GC), and distilling to obtain methanol; (2) salt dissolving: adding water to the crude product to dissolve salt; (3) heating reflux: heating for 3 to 5 hours to reach 95 to 105 DEG C; (4) standing and layering to obtain an oil layer and a water layer; (5) rectifying: performing reduced pressure distillation to the oil layer to obtain finished 1,4-bis(methoxymethyl)-benzene and a byproduct finished p-tolualdehyde. The novel process is simple, convenient and stable in operation process, active substance can be fully utilized and the generation of three wastes (waste gas, waste water, and industrial residue) is reduced in the process of producing the p-xylylene dichloride.
Owner:QIANJIANG XINYIHONG ORGANIC CHEM
Selenite ion type compound and preparation method and application thereof
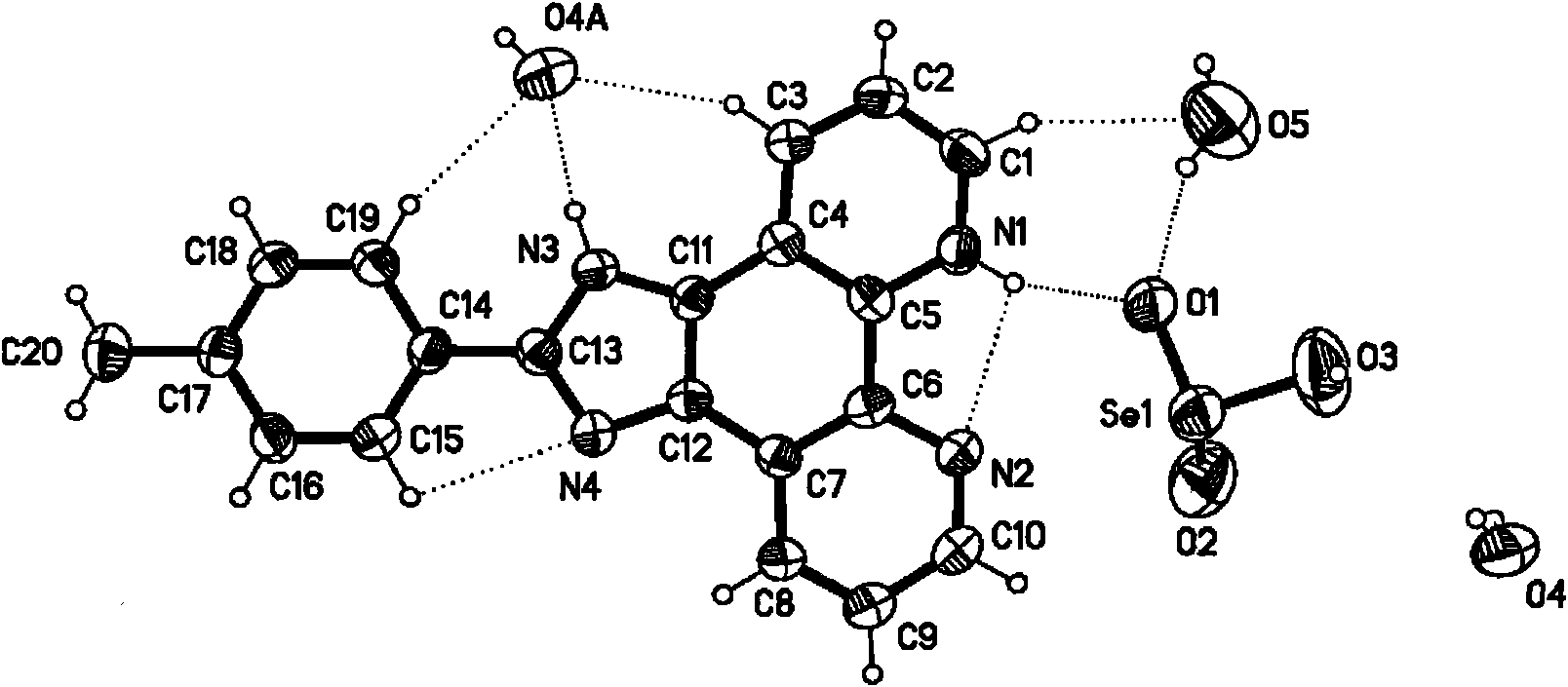
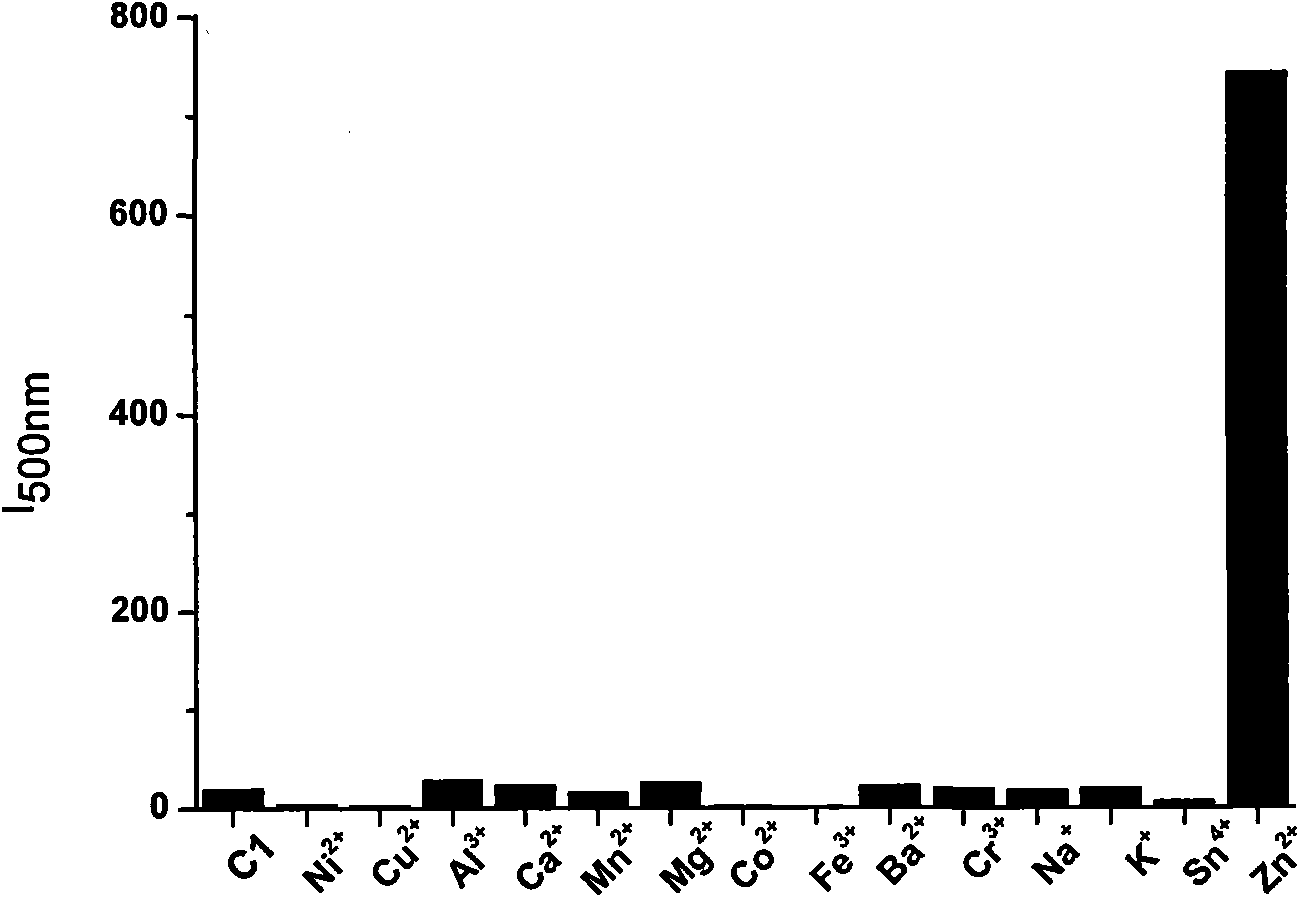
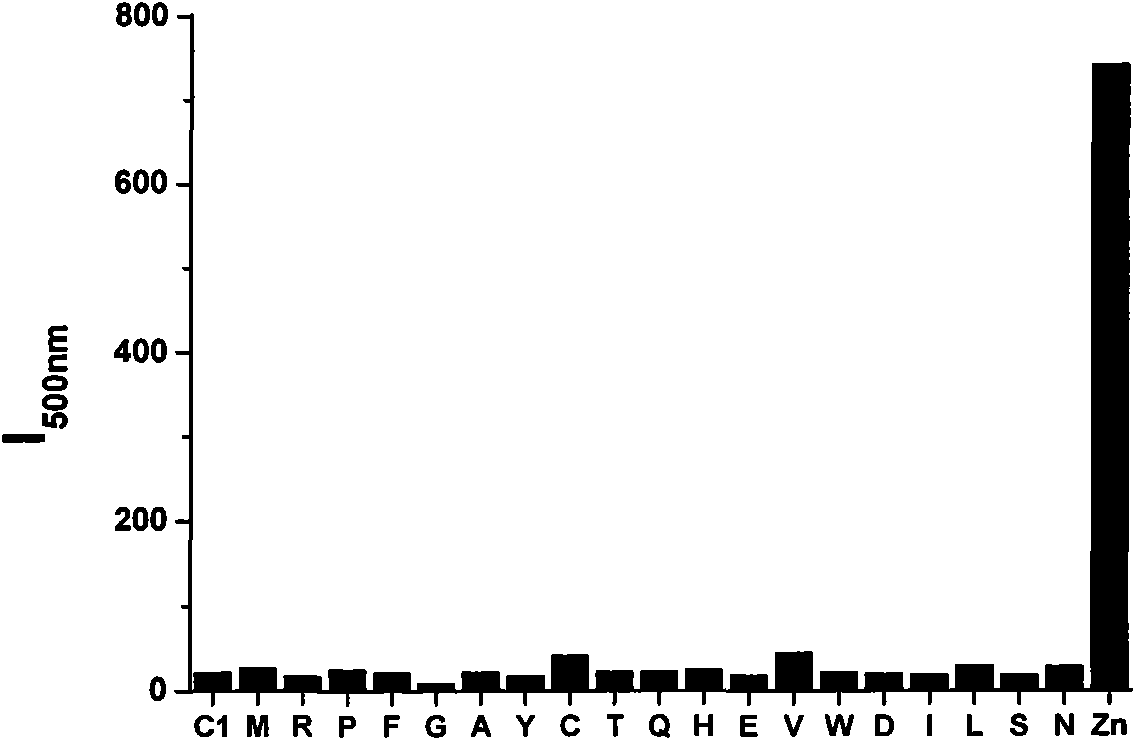
InactiveCN101830901AAvoid damageDetection has no effectOrganic chemistryFluorescence/phosphorescenceFluorescenceChemical compound
The invention provides a selenite ion type compound. The molecular formula of the selenite ion type compound is C20H16N4O3Se. The preparation method comprises the following steps of: preparing 2-(4-tolyl) imidazole [4, 5-f] [1, 10] phenanthroline from phenanthroline dione and p-tolualdehyde; dissolving the 2-(4-tolyl) imidazole [4, 5-f] [1, 10] phenanthroline in dioxane, and slowly adding the solution into a dioxane solution in which selenium dioxide is dissolved at 50 to 65 DEG C, and stirring for at least half an hour at 80 DEG C, cooling, filtering and drying in vacuum to obtain the product. The compound can specifically identify zinc ions, and can be applied to detection of zinc ions in organisms and fluorescence labeling of zinc ions in cells.
Owner:SHANXI UNIV
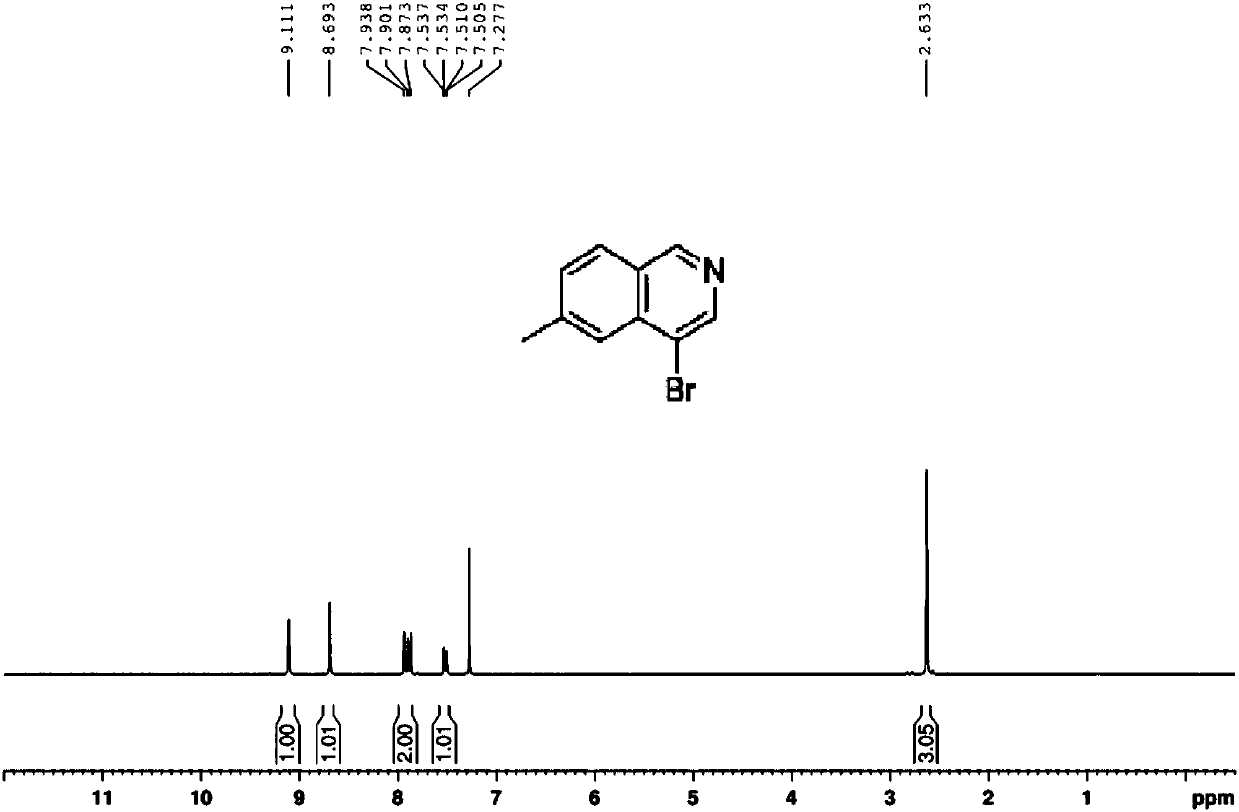
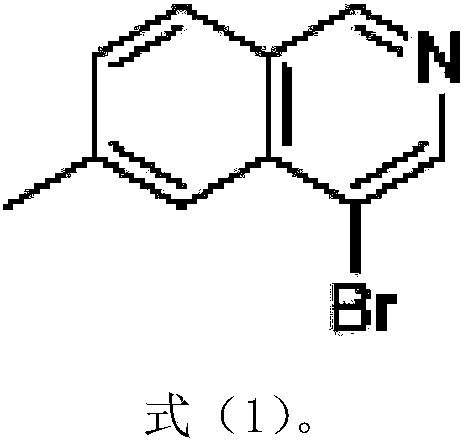
Preparation method of 6-methyl-4-bromo-isoquinoline
InactiveCN108033909AFew synthetic stepsStrong invention ideaOrganic chemistrySodium hydroxideP-tolualdehyde
The present invention relates to a synthesis method of 6-methyl-4-bromo-isoquinoline. The synthesis method comprises the following steps: using amino acetaldehyde dimethyl acetal and p-tolualdehyde asraw materials, and carrying out a reaction for forming 2,2-methyl-N-(4-methoxy phenyl alkenyl)ethylamine; then adding methyl chloroacetate and trimethyl phosphite to obtain 2,2-dimethoxyethyl((dimethoxyphosphoryl)(p-methylphenyl)methyl)ethyl carbamate; further adding titanium tetrachloride for a reaction, after the reaction traced by a TLC method is completed, further adding a 2N sodium hydroxidewater solution for reaction, then adding diatomite, carrying out stirring and suction filtration, carrying out extraction on the water phase and the filter cake with ethyl acetate, carrying out drying and rotary evaporateion, and carrying out column chromatography purification by using a sufficient amount of silica gel columns of 100-200 meshes to obtain 6-methyl isoquinoline; and adding the 6-methyl isoquinoline into carbon tetrachloride, then adding N-bromo-succinimide, carrying out heating and refluxing for 2-5 hours, concentrating the reaction mixture, carrying out column chromatography with silica gel columns of 100-200 meshes, using petroleum ether PE and ethyl acetate EA as eluants, and carrying out recrystallization with excess petroleum ether to obtain the final product 4-bromo-6-methyl-isoquinoline. The method has the advantages of clear steps, less waste, high yield, raw material conservation and easy operation.
Owner:北京六合宁远医药科技股份有限公司
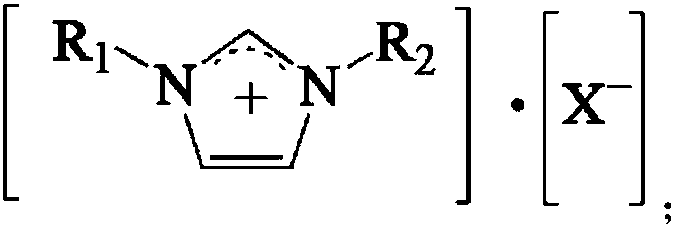
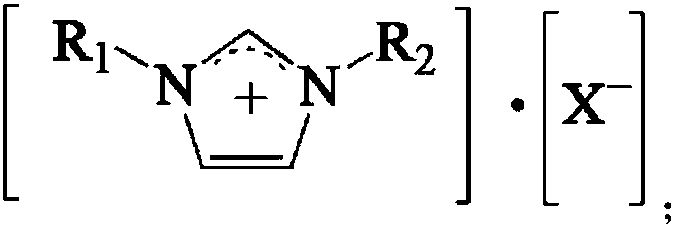
Catalyst for synthesizing p-tolualdehyde
ActiveCN109647508ABeneficial technical effectOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by carbon monoxide reactionRare earthToluene
The invention relates to a catalyst for synthesizing p-tolualdehyde. The problems of low conversion rate of toluene and low yield of p-tolualdehyde in the prior art are mainly solved in the invention.The catalyst for synthesizing p-tolualdehyde comprises an ionic liquid and a rare earth salt, and the ionic liquid is selected from ionic liquids having a structural formula shown in the description;and in the structural formula, R1 and R2 are independently selected from C1-C4 alkyl groups, and X is at least one selected from PF6, SbF6, AlCl4, BF4, PF4, CF3COO, CF3SO3 and (CF3SO2)2N. The catalyst well solves the technical problems, and can be used in the industrial production of p-tolualdehyde.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for synthesizing 3-(4-methyl benzene methylene) camphor
InactiveCN105985228AThe method is simpleReduce wasteOrganic compound preparationCarbonyl compound preparationLiquid wasteFiltration
The invention discloses a method for synthesizing 3-(4-methyl benzene methylene) camphor. The method comprises the following steps that firstly, camphor, a reaction solvent and an immobilized catalyst KOH / Al2O3 are added into a reaction container; secondly, the temperature is raised to a reaction temperature, and p-tolualdehyde is added dropwise; thirdly, after the reaction is finished, the immobilized catalyst KOH / Al2O3 is removed through filtration, washing is carried out through saturated brine, drying is carried out through magnesium sulfate, and the reaction solvent is recycled at normal pressure; fourthly, recrystallization is carried out through an ethanol solution, and by means of drying treatment, the product is obtained. The method for synthesizing the 3-(4-methyl benzene methylene) camphor through the immobilized catalyst KOH / Al2O3 in a catalyzing mode is simple and easy to implement, little liquid waste is caused, the catalyst is recycled easily, and the yield can reach 85%. The purity of the 3-(4-methyl benzene methylene) camphor prepared through the recrystallization post-treatment method can reach 99.5%.
Owner:NANJING UNIV OF SCI & TECH
Synthetic method of procarbazine
InactiveCN110156629AHigh yieldReduce usageHydrazine preparationOrganic compound preparationStrong acidsSolvent
The invention discloses a synthetic method of procarbazine (Pcb). The synthetic method comprises following steps: p-tolualdehyde is taken as an initial raw material, dibromocyanuric acid and isopropylamine are added, reaction is carried out at room temperature so as to obtain p-methyl benzoyl isopropylamine; p-methyl benzoyl isopropylamine is dissolved in an organic reagent, N-bromo-succinimide and an initiator are added, heating reflux is carried out, after reaction, the solvent is removed, acetonitrile and a hydrolysis promoter are added, heating reflux is carried out so as to obtain p-formyl benzoyl isopropylamine; p-formyl benzoyl isopropylamine and methylhydrazine sulfate are dissolved in an organic reagent, triethylamine is added for reaction, and the solvent is subjected to rotary drying, sodium cyanoborohydride is added, an obtained reaction system is heated to room temperature, reaction is carried out for more than one night so as to obtain Pcb. According to the synthetic method, bromination is carried out firstly, and then hydrolysis is carried out, p-methyl benzoyl isopropylamine is converted into p-formyl benzoyl isopropylamine, using of strong oxidizing agent and strong acid is avoided, the synthetic method is friendly to the environment, the yield is high, and the total yield of the three steps is 52.9%.
Owner:广州药本君安医药科技股份有限公司
Method for synthesizing p-tolualdehyde
ActiveCN109651124ABeneficial technical effectOrganic compound preparationPreparation by carbon monoxide reactionRare earthCarbonylation
The invention relates to a method for synthesizing p-tolualdehyde. The problems of low conversion rate of toluene and low yield of p-tolualdehyde in the prior art are mainly solved in the invention. The method for synthesizing p-tolualdehyde is characterized in that toluene and CO undergo a carbonylation reaction under the catalysis of a catalyst to obtain the p-tolualdehyde, wherein the catalystcomprises an ionic liquid and a rare earth salt, and the ionic liquid is selected from ionic liquids having a structural formula shown in the description; and in the structural formula, R1 and R2 areindependently selected from C1-C4 alkyl groups, and X is at least one selected from PF6, SbF6, AlCl4, BF4, PF4, CF3COO, CF3SO3 and (CF3SO2)2N. The method well solves the technical problems, and can beused in the industrial production of p-tolualdehyde.
Owner:CHINA PETROLEUM & CHEM CORP +1
Method for producing p-xylene and/or p-tolualdehyde
InactiveUS9024074B2High yieldShort processOrganic compound preparationHydrocarbons from unsaturated hydrocarbon additionGas phaseAcrolein
Disclosed is a method for producing p-xylene and / or p-tolualdehyde with high yield through a short process using biomass resource-derived substances as raw materials. The method for producing p-xylene and / or p-tolualdehyde of the present invention comprises: a cyclization step of producing 4-methyl-3-cyclohexenecarboxaldehyde from isoprene and acrolein; and an aromatization step of producing p-xylene and / or p-tolualdehyde from 4-methyl-3-cyclohexenecarboxaldehyde by gas-phase flow reaction using a catalyst(s).
Owner:TORAY IND INC
Preparation method of p-methyl cinnamic acid
InactiveCN106631754AAvoid transportAvoid preparationOrganic compound preparationCarboxylic acid esters preparationAcetic acidAlcohol
The invention belongs to the technical field of medicine synthesis and in particular relates to a preparation method of an ozagrel key intermediate, namely p-methyl cinnamic acid. The method comprises the following steps: taking p-tolualdehyde as a raw material and alcohol as a reaction solvent; reacting under the protection of nitrogen gas and under a heating condition of sodium alcoholate and / or a sodium alcoholate alcohol solution and acetate; after reacting, concentrating until an oily product is obtained; adding an alkali solution and reacting and hydrolyzing; adding an acid for acidifying and carrying out acid regulation to obtain the p-methyl cinnamic acid. The preparation method provided by the invention has the advantages of moderate reaction conditions, simplicity in operation, high yield, high product purity, low raw material cost and the like, has the advantages of environment friendliness, greenness and environment protection, and is suitable for industrial production.
Owner:山东诚汇双达药业有限公司
A new process for the manufacture of p-xylylene dimethyl ether
ActiveCN103288604BShorten the production cycleEasy to operateCarbonyl compound preparation by hydrolysisEther preparationSodium methoxideXylylene
The invention provides a novel process of a 1,4-bis(methoxymethyl)-benzene preparation method. The novel process comprises the following steps of: (1) reaction distillation: heating low-boiling leftovers containing p-xylylene dichloride to reach the temperature above 110 DEG C, pumping into a reaction kettle under vacuum; adding sodium methoxide solution to react and distil at temperature below 80 to 100 DEG C, stopping reaction to obtain a crude product while the content of the p-xylylene dichloride residual in a reactant accounts for 0.05 to 0.5% (GC), and distilling to obtain methanol; (2) salt dissolving: adding water to the crude product to dissolve salt; (3) heating reflux: heating for 3 to 5 hours to reach 95 to 105 DEG C; (4) standing and layering to obtain an oil layer and a water layer; (5) rectifying: performing reduced pressure distillation to the oil layer to obtain finished 1,4-bis(methoxymethyl)-benzene and a byproduct finished p-tolualdehyde. The novel process is simple, convenient and stable in operation process, active substance can be fully utilized and the generation of three wastes (waste gas, waste water, and industrial residue) is reduced in the process of producing the p-xylylene dichloride.
Owner:QIANJIANG XINYIHONG ORGANIC CHEM
Production method of p-tolualdehyde
InactiveCN106083552AAchieve productivityTo achieve the purpose of comprehensive utilizationCarbonyl compound preparation by hydrolysisHalogenated hydrocarbon preparationXylyleneAcid water
The invention relates to a production method of p-tolualdehyde. The method comprises the steps of: pumping a p-xylylene dichloride low-boiling-point compound containing p-methylbenzylidene into a rectifying tower, collecting a p-methylbenzylidene finished product and p-xylylene dichloride finished product fractions; putting the p-methylbenzylidene finished product into an acidolysis reaction kettle, and adding a catalyst and water at the same time; at the end of acidolysis, conveying the lower layer acid water to an acid water storage tank, and pumping an upper layer oil phase into an alkaline hydrolysis kettle; putting Na2CO3 into the alkaline hydrolysis kettle to carry out alkaline hydrolysis reaction, and then pumping the upper layer oil phase into a coarse distillation tower; conducting coarse distillation: subjecting the upper layer oil phase to pressure reduced distillation; performing rectification: putting the collected overhead distillate into the rectifying tower to perform pressure reduced rectification, and collecting 90-115DEG C / 30mmHg fractions, thus obtaining a p-tolualdehyde finished product with purity of 99%. The production method of p-tolualdehyde provided by the invention can fully recycle p-methylbenzylidene, realizes zero discharge of process wastewater, has high yield, and can lower the production cost.
Owner:QIANJIANG XINYIHONG ORGANIC CHEM
Refining method of terephthalaldehyde
InactiveCN109879736AHigh purityHigh yieldCarbonyl compound separation/purificationEvaporationDissolution
The invention discloses a refining method of terephthalaldehyde, which comprises the following steps: evaporating a terephthalaldehyde crude product under a vacuum or normal pressure condition, and after impurities are removed, taking paraxylene as a solvent, and conducting dissolving, filtering, cooling crystallizing, filtering and drying to obtain a terephthalaldehyde refined product. The methodhas the advantages that only a reaction raw material paraxylene is used as the solvent, the technology process is environmentally friendly, impurities such as p-tolualdehyde are removed through evaporation, then the refined terephthalaldehyde product is obtained through paraxylene dissolution and crystallizing processes, the product purity is high, the yield is high, the product purity reaches 99% or above, and the yield reaches 90% or above.
Owner:SINOPEC YANGZI PETROCHEM +1
Synthetic method of alpha,alpha,alpha-trifluoro-p-tolualdehyde
InactiveCN109111354AReduce dosageProduced noOrganic-compounds/hydrides/coordination-complexes catalystsCarbonyl compound preparation by hydrolysisFormaldehyde productGas phase
The invention discloses a preparation method of alpha,alpha,alpha-trifluoro-p-tolualdehyde. Under the action of a self-made catalyst, alpha,alpha,alpha-trifluoro-p-benzyl bichloride and water generatea hydrolysis reaction, after the reaction is ended, reaction materials are washed and layered, the benzaldehyde product with 98.5% or above of gas phase content is obtained by purifying a benzaldehyde crude product, and the total product yield is up to 96% or above. The catalyst is recovered through vacuum drying of a water layer, and can be repeatedly used. Hydrogen chloride generated in the process is absorbed with water and can be prepared into hydrochloric acid. The method provided by the invention has excellent characteristics of simple process and environment protection such as less three wastes, thereby being more suitable for industrial mass production.
Owner:阜新汉道化工有限责任公司
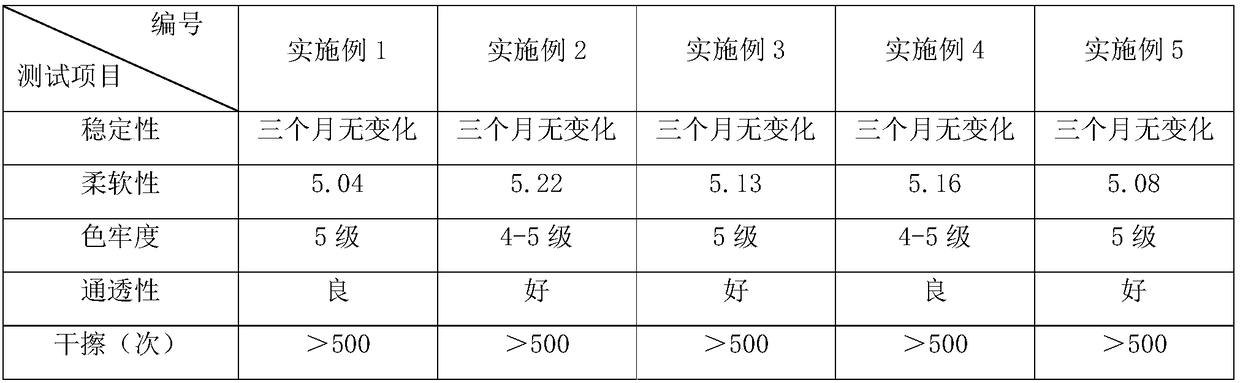
Leather care agent and preparation method thereof
InactiveCN108866260AStay flexibleMaintain colorLeather surface finishingDimethyl siloxaneP-tolualdehyde
The invention discloses a leather care agent and a preparation method thereof. The leather care agent comprises, by weight part, 3-8 parts of methacrylic acid, 2-5 parts of butyl acrylate, 3-7 parts of an excoecaria cochinchinensis lour extracting solution, 4-7 parts of a pomegranate extracting solution, 4-8 parts of a lemongrass extracting solution, 4-10 parts of sulfated castor oil, 2-6 parts ofsodium pyrosulfite, 2-7 parts of hexadecanol polyoxyethylene ether, 1-5 parts of polydimethylsiloxane, 3-7 parts of toluene diisocynate, 4-8 parts of p-tolualdehyde, 2-5 parts of polymethylhydrosiloxane, 2-6 parts of polyurethane, 1-5 parts of ethylene glycol monobutyl ether and 6-14 parts of water. By means of the leather care agent, leather can be fully protected while the leather is softened and the hand feeling is improved; the leather is not prone to aging or fading; and harmful substances can be cleared off, and dust removal and mold prevention are achieved.
Owner:深圳市艾贝比品牌管理咨询有限公司
Refining method of terephthalaldehyde
PendingCN109879737AHigh refining yieldSimple processCarbonyl compound separation/purificationFormaldehyde productSolvent
The invention discloses a refining method of terephthalaldehyde, which comprises the following steps: evaporating a terephthalaldehyde crude product under a vacuum condition, removing impurities, andwith water as a solvent, sequentially conducting dissolving, filtering, crystallizing, filtering and drying to obtain a terephthalaldehyde refined product. The method has the advantages that no solvent is used; the technological process is environmentally friendly, impurities such as p-tolualdehyde are removed through vacuum evaporation, then the refined terephthalaldehyde product is obtained through dissolving and crystallizing processes with water as a solvent, the method has the characteristics of high purity and high yield, the product purity reaches 99% or above, and the yield reaches 90%or above.
Owner:SINOPEC YANGZI PETROCHEM +1
Production method of p-tolualdehyde
InactiveCN106365963AAchieve productivityTo achieve the purpose of comprehensive utilizationCarbonyl compound preparation by hydrolysisHalogenated hydrocarbon preparationXylyleneAcid water
The invention relates to a production method of p-tolualdehyde. The production method comprises the following steps: sucking p-methylbenzylidene-containing p-xylylene dichloride into a rectifying tower under a low boiling condition, and collecting a p-methylbenzylidene finished product and a p-xylylene dichloride finished product fraction; feeding the p-methylbenzylidene finished product into an acidolysis reaction kettle, and adding a catalyst and water at the same time; after acidolysis is completed, conveying acid water on the lower layer into an acid water storage tank, and sucking an oil phase on the upper layer into an alkaline hydrolysis kettle; feeding Na2CO3 into the alkaline hydrolysis kettle, and sucking an oil phase on the upper layer into a crude distillation tower after alkaline hydrolysis reaction is completed; performing coarse distillation: performing decompression distillation on the oil phase on the upper layer; performing rectification: feeding collected fractions at the top of the tower into the rectifying tower for decompression rectification, and collecting a fraction at the temperature of 100 to 110 DEG C per 30 mmHg to obtain a p-tolualdehyde finished product with the purity of 99 percent. According to the production method of the p-tolualdehyde, the p-methylbenzylidene can be fully recycled, and zero discharge of process wastewater can be realized; the yield is high; the production cost can be reduced.
Owner:枣阳市众成化工有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
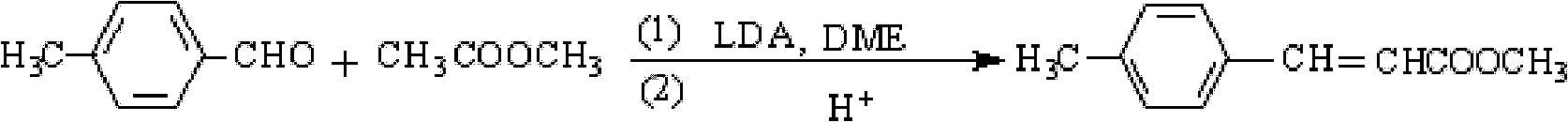
![Oxaphosphaphenanthrene fire retardant containing cyclotriphosphazene and cup [4] aromatic ring structures as well as preparation method and application of oxaphosphaphenanthrene fire retardant Oxaphosphaphenanthrene fire retardant containing cyclotriphosphazene and cup [4] aromatic ring structures as well as preparation method and application of oxaphosphaphenanthrene fire retardant](https://images-eureka.patsnap.com/patent_img/0eff8d6b-4fdc-4cd0-a6b6-fa996d990bb6/BDA0000569190440000011.PNG)
![Oxaphosphaphenanthrene fire retardant containing cyclotriphosphazene and cup [4] aromatic ring structures as well as preparation method and application of oxaphosphaphenanthrene fire retardant Oxaphosphaphenanthrene fire retardant containing cyclotriphosphazene and cup [4] aromatic ring structures as well as preparation method and application of oxaphosphaphenanthrene fire retardant](https://images-eureka.patsnap.com/patent_img/0eff8d6b-4fdc-4cd0-a6b6-fa996d990bb6/BDA0000569190440000021.PNG)
![Oxaphosphaphenanthrene fire retardant containing cyclotriphosphazene and cup [4] aromatic ring structures as well as preparation method and application of oxaphosphaphenanthrene fire retardant Oxaphosphaphenanthrene fire retardant containing cyclotriphosphazene and cup [4] aromatic ring structures as well as preparation method and application of oxaphosphaphenanthrene fire retardant](https://images-eureka.patsnap.com/patent_img/0eff8d6b-4fdc-4cd0-a6b6-fa996d990bb6/BDA0000569190440000022.PNG)