Novel process of 1,4-bis(methoxymethyl)-benzene preparation method
The technology of a p-xylylene dimethyl ether and a manufacturing method is applied in the new process field of the p-xylylene dimethyl ether manufacturing method, and can solve the problems of unenvironmental operators, easy generation of flue gas, air pollution and the like, Achieve the effect of reducing accidents and side reactions, simplifying the operation process and simplifying the operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
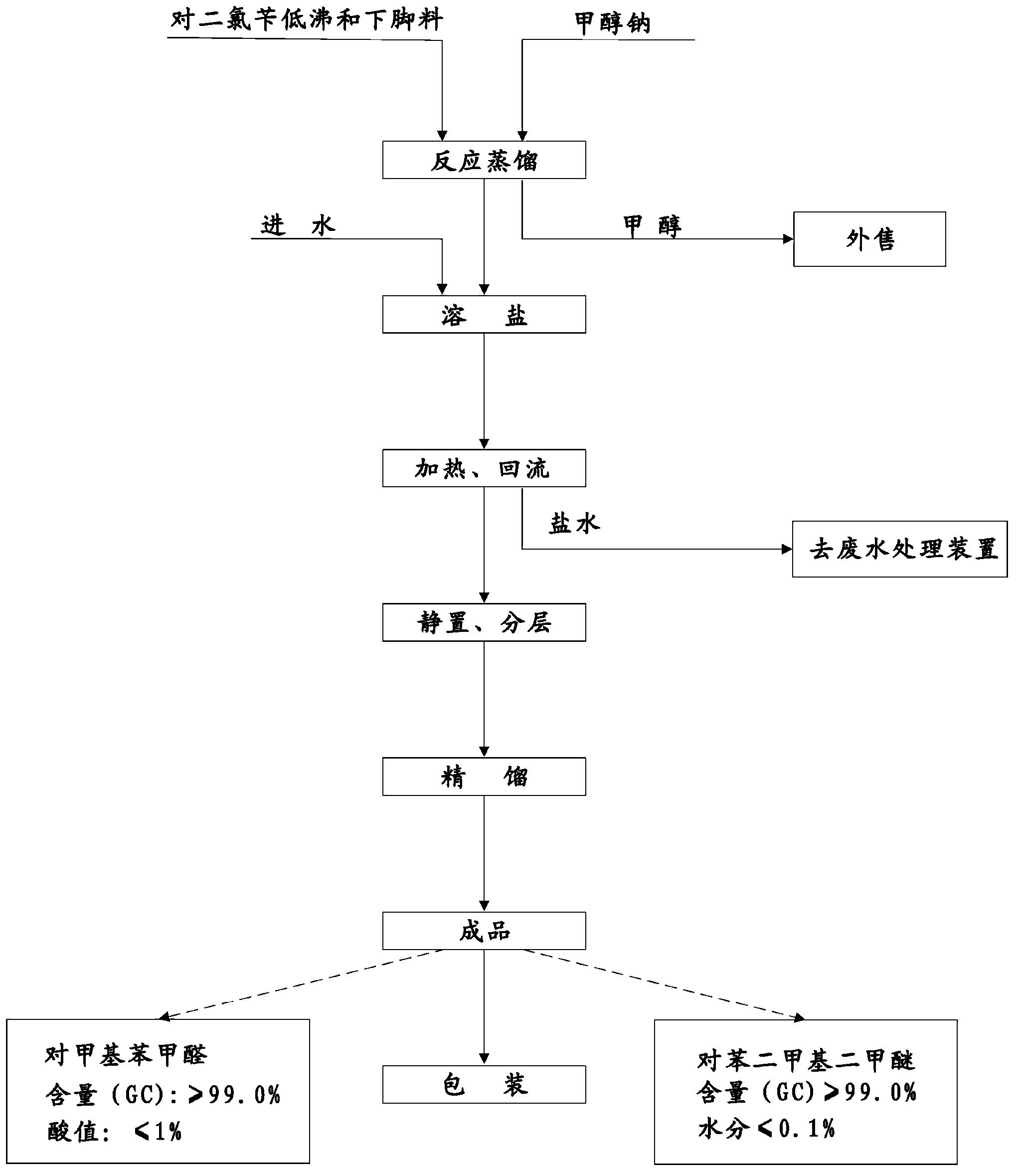
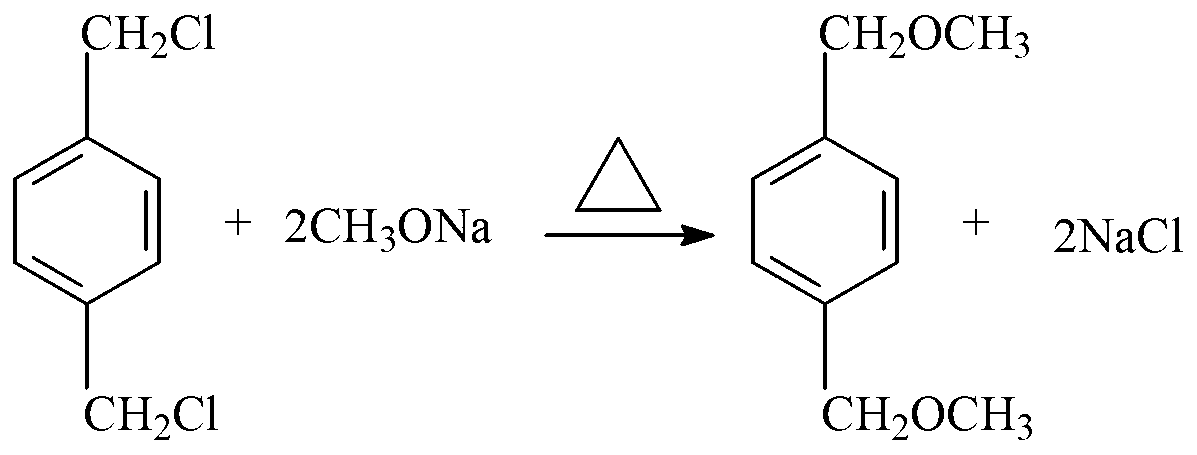
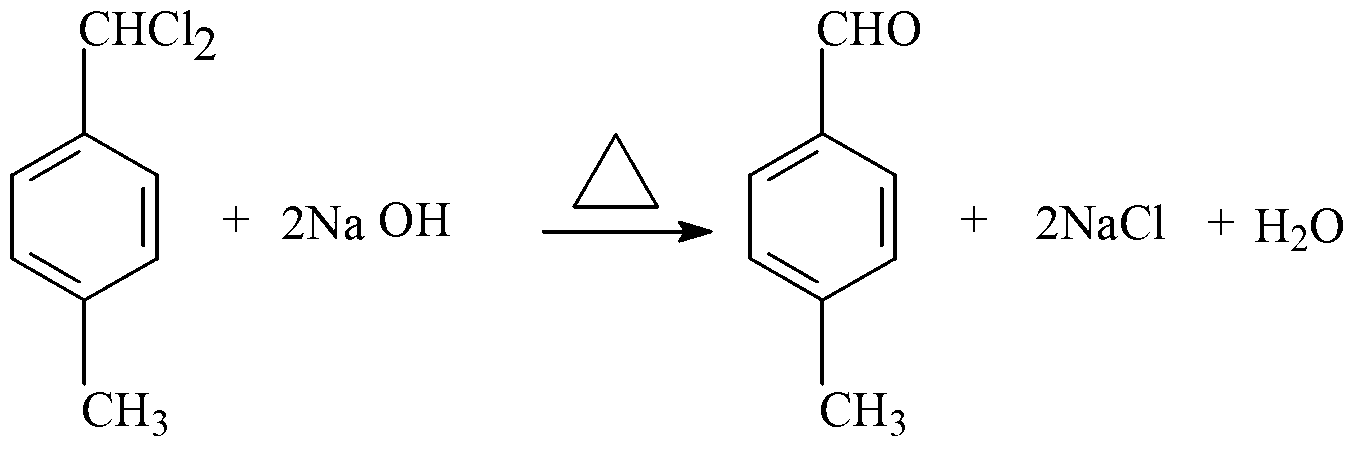
[0033] (1) see figure 1 As shown in the process flow diagram of the present invention, take p-dichlorobenzyl low boiling and leftovers 175g (45% (GC) p-dichlorobenzyl, 40% (GC) p-methylbenzylidene), drop at a temperature of 90°C Add 502.6g (2.6mol) of sodium methoxide solution, when the content of residual dichlorobenzyl in the reactant is 0.05% to 0.5% (GC), the reaction can be terminated, and the methanol in the reaction crude product is distilled out at the same time, methanol can be used For sale. Then, add water to the crude product to dissolve the salt; heat the solution to 105°C, and reflux the reaction for 2 hours; put the obtained reaction liquid into layers, remove the lower water layer (that is, brine), and the brine can be moved to a waste water treatment device for treatment . Then the oil layer is subjected to vacuum distillation, and the fraction of 130-140°C / 30mmHg is collected to obtain the finished p-xylylene dimethyl ether product and the fraction of 110-1...
Embodiment 2
[0035] see figure 1 As shown in the process flow diagram of the present invention, take p-dichlorobenzyl low boiling and leftovers 175g (45% (GC) p-dichlorobenzyl, 40% (GC) p-methylbenzylidene), drop at a temperature of 100 ° C Add 502.6g (2.6mol) of sodium methoxide solution, when the content of residual dichlorobenzyl in the reactant is 0.05% to 0.5% (GC), the reaction can be terminated, and the methanol in the reaction crude product is distilled out at the same time, methanol can be used For sale. Then, add water to the crude product to dissolve the salt; heat the solution to 105°C, and reflux the reaction for 4 hours; put the obtained reaction liquid into layers, remove the lower water layer (that is, brine), and the brine can be moved to a waste water treatment device for treatment . Then the oil layer is subjected to vacuum distillation, and the fraction of 130-140°C / 30mmHg is collected to obtain the finished p-xylylene dimethyl ether product and the fraction of 110-11...
Embodiment 3
[0037] see figure 1 As shown in the process flow diagram of the present invention, take p-dichlorobenzyl low boiling and leftovers 175g (45% (GC) p-dichlorobenzyl, 40% (GC) p-methylbenzylidene), drop at a temperature of 80 ° C Add 432g (2.4mol) of sodium methoxide solution, and the reaction can be terminated when the residual content of dichlorobenzyl in the reactant is 0.05% to 0.5% (GC). sale. Then, add water to the crude product to dissolve the salt; heat the solution to 105°C, and reflux the reaction for 4 hours; put the obtained reaction liquid into layers, remove the lower water layer (that is, brine), and the brine can be moved to a waste water treatment device for treatment . Then the oil layer is subjected to vacuum distillation, and the fraction of 130-140°C / 30mmHg is collected to obtain the finished p-xylylene dimethyl ether product and the fraction of 110-115°C / 30mmHg p-tolualdehyde to obtain the p-xylylene dimethyl The finished product of ether is 74.2g, the fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com