Aromatic diamine and polyimide containing tolyl and non-coplanar structure and their preparation methods
A non-coplanar, aromatic diamine technology, applied in the field of polyimide, can solve the problems of restricting the industrial production of diamine monomers containing large side groups, few types of diamine monomers containing large side groups, and complicated purification methods. , to achieve the effect of favorable diffusion and dissolution, excellent solubility, and easy safe operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of diamine monomer:
[0058] (1) Add the mixture of 24.24g (0.2mol) 2,6-dimethylaniline and 150mL distilled water into a 250mL three-neck round-bottomed flask equipped with magnetic stirring, reflux condenser, constant pressure dropping funnel, nitrogen inlet and outlet, and stir at room temperature for 0.5h ;
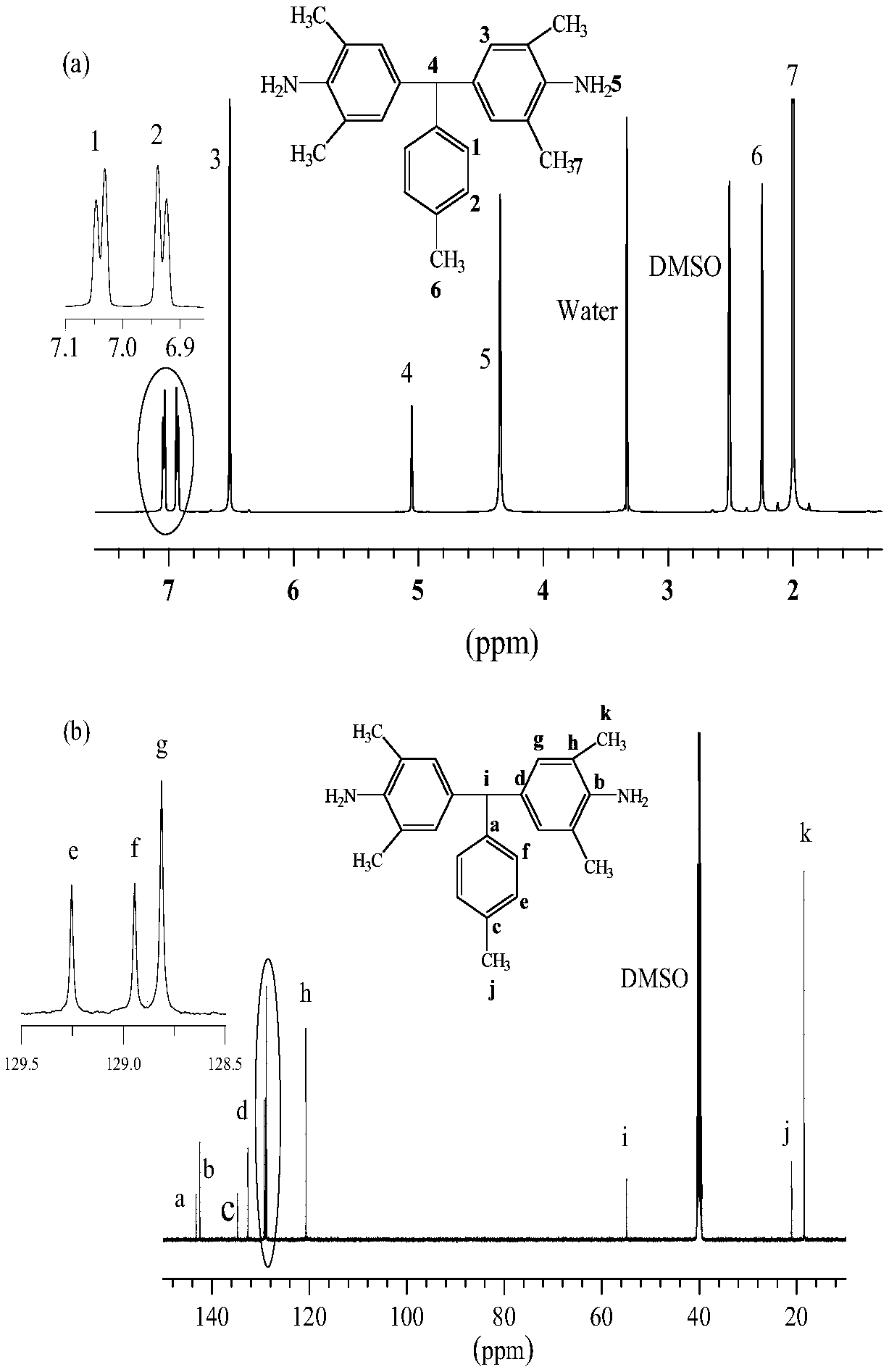
[0059] (2) Pass in the inert gas nitrogen, then add dropwise a mixture of 20mL concentrated hydrochloric acid (12mol / L) and 20mL distilled water, drop it within 1h; then raise the temperature to 110°C, add dropwise 12.36g (0.101mol) 4-methylbenzene Formaldehyde, drop it within 1h; maintain the temperature of 110°C for 12h, cool to room temperature; pour the product into excess methanol, add 200mL sodium hydroxide (15wt%) to form a white precipitate, filter and dry, wash with distilled water several times, and use the crude product Recrystallization of n-butanol yielded polymer grade aromatic diamine monomer (yield 90%, purity 95%). The H NMR and C ...
Embodiment 2
[0066] Preparation of diamine monomer:
[0067] (1) Add 24.24g (0.2mol) of 2,6-dimethylaniline and 120mL of distilled water into a 250mL three-neck round bottom flask equipped with magnetic stirring, reflux condenser, constant pressure dropping funnel, nitrogen inlet and outlet, and stir for 2 hours at room temperature;
[0068] (2) Pass in the inert gas nitrogen, then add dropwise a mixture of 20mL concentrated hydrochloric acid (12mol / L) and 20mL distilled water, drop it within 1h; then raise the temperature to 90°C, add dropwise 12.85g (0.105mol) 4-methylbenzene Formaldehyde, drop it within 1h; maintain the temperature at 90°C for 8h, cool to room temperature; pour the product into excess methanol, add 200mL sodium hydroxide (15wt%) to form a white precipitate, filter and dry, wash with distilled water several times, and use the crude product Recrystallization of n-butanol yielded polymer grade aromatic diamine monomer (yield 80%, purity 90%).
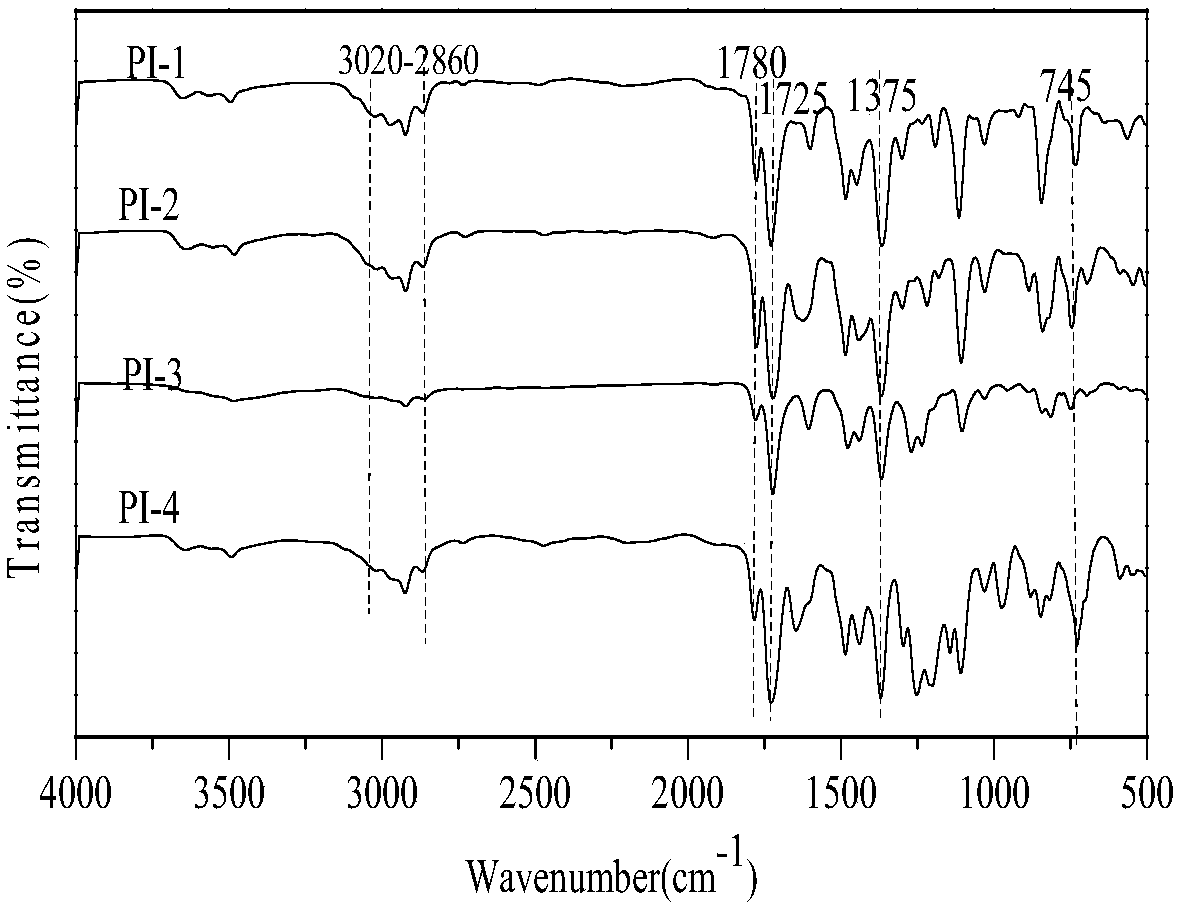
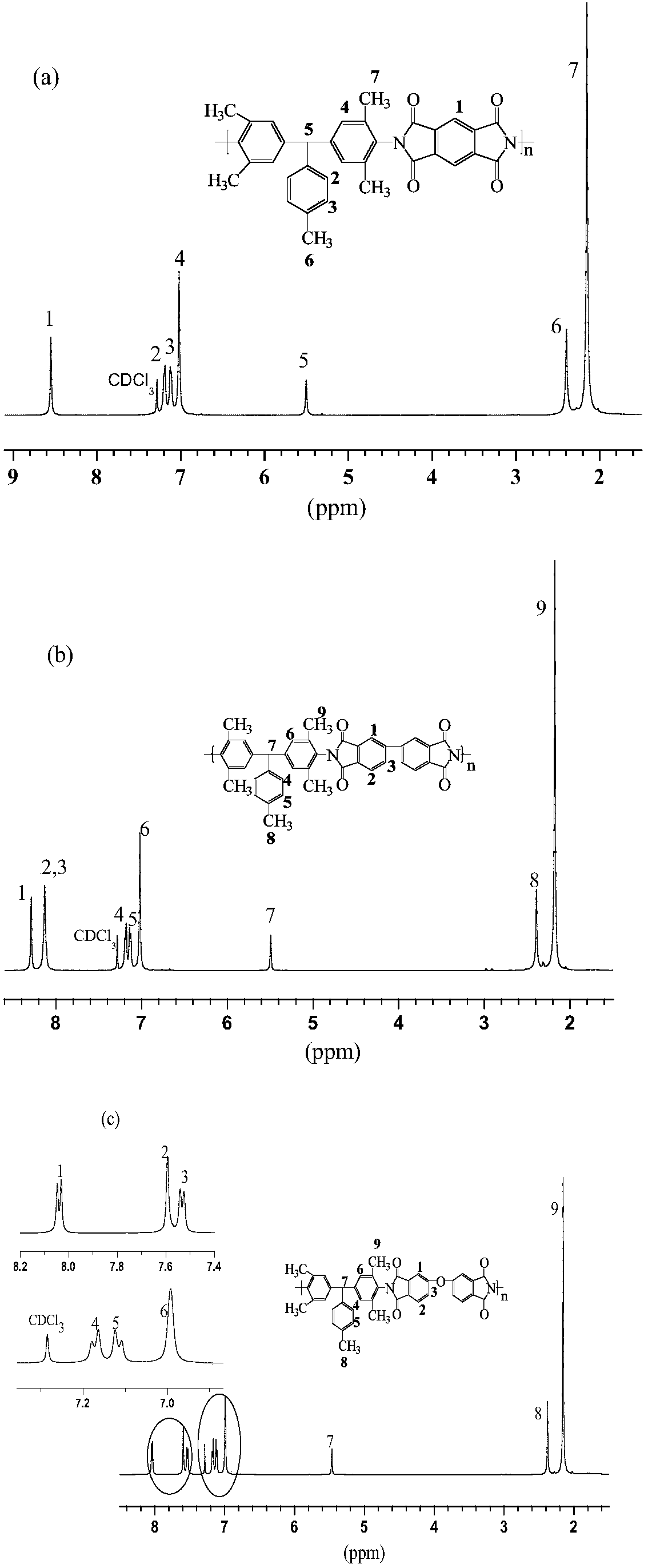
[0069] Synthesis of polyimi...
Embodiment 3
[0073] Preparation of diamine monomer:
[0074] (1) Add 24.24g (0.2mol) of 2,6-dimethylaniline and 100mL of distilled water into a 250mL three-neck round bottom flask equipped with magnetic stirring, reflux condenser, constant pressure dropping funnel, nitrogen inlet and outlet, and stir for 1 hour at room temperature;
[0075] (2) Pass in the inert gas nitrogen, then add dropwise a mixture of 20mL concentrated hydrochloric acid (12mol / L) and 20mL distilled water, drop it within 1h; then raise the temperature to 80°C, add dropwise 13.46g (0.11mol) 4-methylbenzene Formaldehyde, drop it within 1h; maintain the temperature of 80°C for 12h, cool to room temperature; pour the product into excess methanol, add 200mL sodium hydroxide (15wt%) to form a white precipitate, filter and dry, wash with distilled water several times, and use the crude product Recrystallization of n-butanol yielded polymer grade aromatic diamine monomer (yield 50%, purity 92%).
[0076] Synthesis of polyimid...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Transmittance | aaaaa | aaaaa |
| Molecular weight distribution coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com