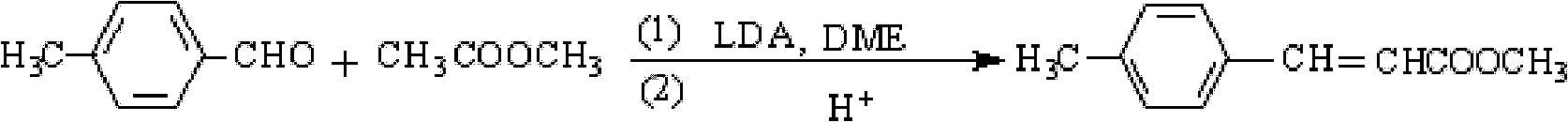Preparation method of methyl 4-methylcinnamate
A technology of methyl cinnamate and p-methylbenzaldehyde, which is applied in the preparation of carboxylate, the preparation of organic compounds, chemical instruments and methods, etc., can solve the problem of not meeting green production requirements, long reaction time, poor production conditions, etc. problems, to achieve the effects of stable and reliable product quality, simple method and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In the three-necked flask, add 9.0g (0.1mol) DME, 5.6g (0.0525mol) LDA and 3.7g (0.05mol) methyl acetate, after stirring for 0.5h under ice-water bath, slowly add 6.3 g (0.0525mol) p-tolualdehyde, keep the temperature below 5°C; after dropping, keep it at 10°C for 3 hours, slowly add 10% sulfuric acid by mass percentage, and adjust the pH of the reaction solution between 6.8 and 7.2 Separate the organic layer, reclaim DME and excessive p-tolualdehyde under reduced pressure to obtain a white solid, then wash and dry to obtain the crude product, recrystallize with absolute ethanol to obtain 6.6g of methyl p-methyl cinnamate, the purity It was 98.7%, and the yield was 73.9% (calculated as methyl acetate).
Embodiment 2
[0026] In the three-necked flask, add 27.0g (0.3mol) DME, 11.8g (0.11mol) LDA and 7.4g (0.1mol) methyl acetate, after stirring for 0.5h under ice-water bath, slowly add 13.2 g (0.11mol) p-tolualdehyde, keep the temperature below 5°C; after dropping, keep it at 7°C for 4 hours, slowly add 10% sulfuric acid by mass percentage, and adjust the pH of the reaction solution between 6.8 and 7.2 Separate the organic layer, reclaim DME and excessive p-tolualdehyde under reduced pressure to obtain a white solid, then wash and dry to obtain the crude product, recrystallize with absolute ethanol to obtain 13.8g of methyl p-methyl cinnamate, the purity It was 99.1%, and the yield was 77.6% (calculated as methyl acetate).
Embodiment 3
[0028] In the three-necked flask, add 47.3g (0.525mol) DME, 19.3g (0.18mol) LDA and 11.1g (0.15mol) methyl acetate, after stirring for 0.5h under ice-water bath, slowly add 21.6 g (0.18mol) p-tolualdehyde, keep the temperature below 5°C; after dropping, keep it at 4°C for 5 hours, slowly add 10% sulfuric acid by mass percentage, and adjust the pH of the reaction solution between 6.8 and 7.2 Separate the organic layer, reclaim DME and excessive p-tolualdehyde under reduced pressure to obtain a white solid, then wash and dry to obtain the crude product, recrystallize with absolute ethanol to obtain 21.9g of methyl p-methyl cinnamate, the purity It was 99.5%, and the yield was 82.4% (calculated as methyl acetate).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com