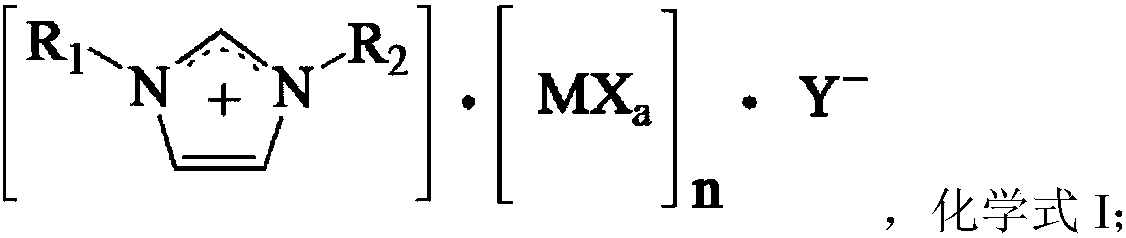Catalyst used for synthesis of p-tolualdehyde
A technology of p-tolualdehyde and catalyst, applied in organic compound/hydride/coordination complex catalyst, physical/chemical process catalyst, organic chemistry, etc., can solve the problem of low selectivity of tolualdehyde
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Catalyst preparation: under nitrogen atmosphere, AlCl 3 (40.0 g, 0.3 mol) was slowly added to N,N-dimethyl imidazole chloride (26.2 g, 0.15 mol), stirred and reacted at 40° C. for 4 h to obtain the catalyst. Elemental analysis: theoretically calculated value C, 15.04; H, 2.27; N, 7.02. Experimental values C, 15.07; H, 2.28; N, 7.01.
[0041] Carbonylation reaction: Add the above catalyst (79.9g, 0.2mol) and toluene (9.2g, 0.1mol) into a 250ml autoclave; replace the air in the kettle with CO gas; raise the temperature to 50°C, keep the reaction pressure at 4.0MPa, and stir at 800rpm , Reacted for 5h to obtain a product mixture containing p-tolualdehyde.
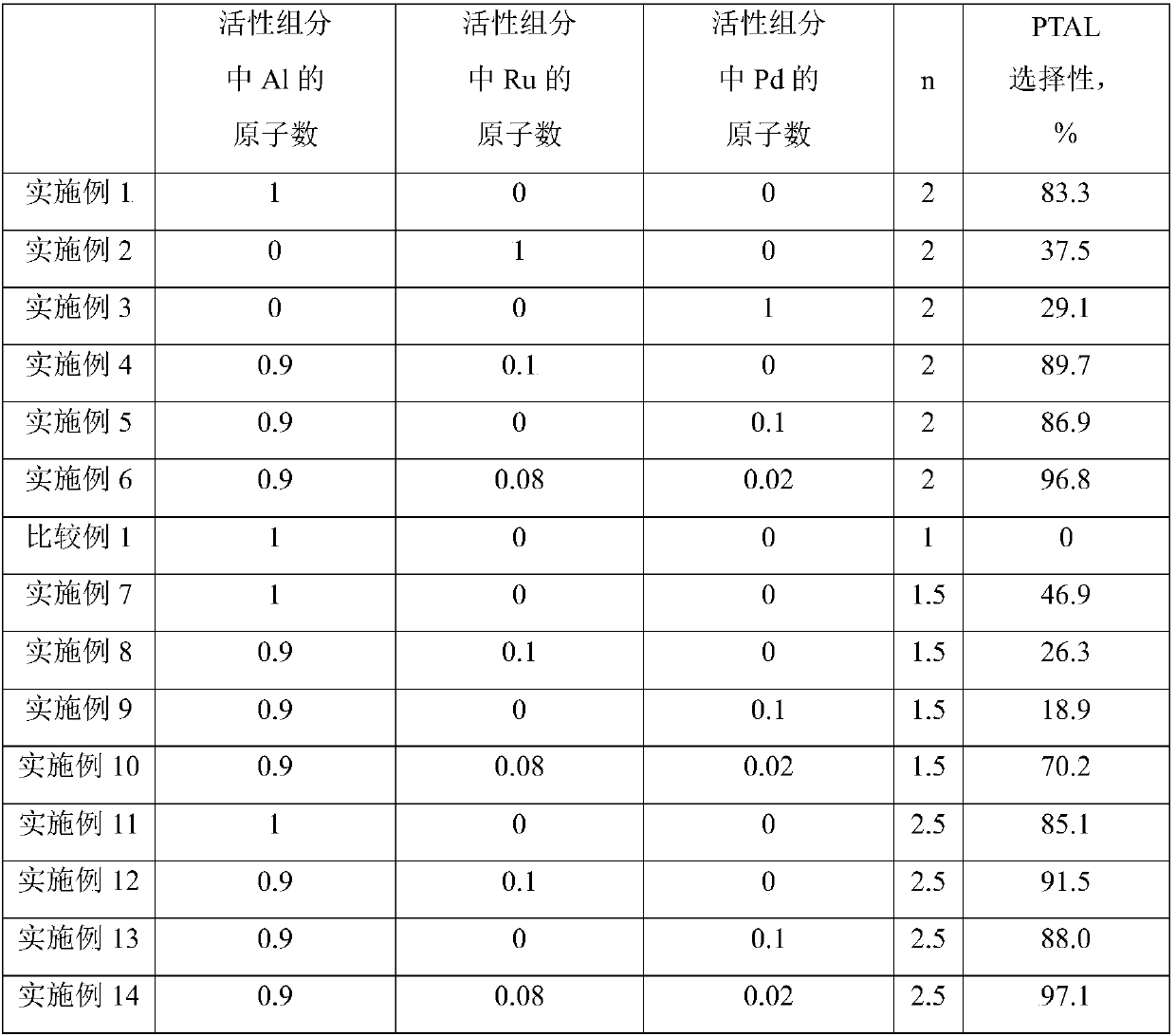
[0042] For the convenience of comparison and illustration, the composition parameters in the catalyst active components and the corresponding selectivity to p-tolualdehyde are listed in Table 1.
Embodiment 2
[0044] Catalyst preparation: under nitrogen atmosphere, RuCl3 (62.2g, 0.3mol) was slowly added into N,N-dimethyl imidazole chloride (26.2g, 0.15mol), stirred and reacted at 40°C for 4h to obtain the catalyst. Elemental analysis: theoretically calculated value C, 10.97; H, 1.66; N, 5.12. Experimental values C, 10.95; H, 1.65; N, 5.13.
[0045] Carbonylation reaction: Add the above catalyst (109.5g, 0.2mol) and toluene (9.2g, 0.1mol) into a 250ml autoclave; replace the air in the kettle with CO gas; raise the temperature to 50°C, keep the reaction pressure at 4.0MPa, and stir at 800rpm , Reacted for 5h to obtain a product mixture containing p-tolualdehyde.
[0046] For the convenience of comparison and illustration, the composition parameters in the catalyst active components and the corresponding selectivity to p-tolualdehyde are listed in Table 1.
Embodiment 3
[0048] Catalyst preparation: under nitrogen atmosphere, PdCl 2 (53.2g, 0.3mol) was slowly added into N,N-dimethyl imidazole chloride (26.2g, 0.15mol), stirred and reacted at 40°C for 4h to obtain the catalyst. Elemental analysis: theoretically calculated value C, 12.33; H, 1.86; N, 5.75. Experimental values C, 12.29; H, 1.86; N, 5.77.
[0049] Carbonylation reaction: Add the above catalyst (97.5g, 0.2mol) and toluene (9.2g, 0.1mol) into a 250ml autoclave; replace the air in the kettle with CO gas; raise the temperature to 50°C, keep the reaction pressure at 4.0MPa, and stir at 800rpm , Reacted for 5h to obtain a product mixture containing p-tolualdehyde.
[0050] For the convenience of comparison and illustration, the composition parameters in the catalyst active components and the corresponding selectivity to p-tolualdehyde are listed in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com