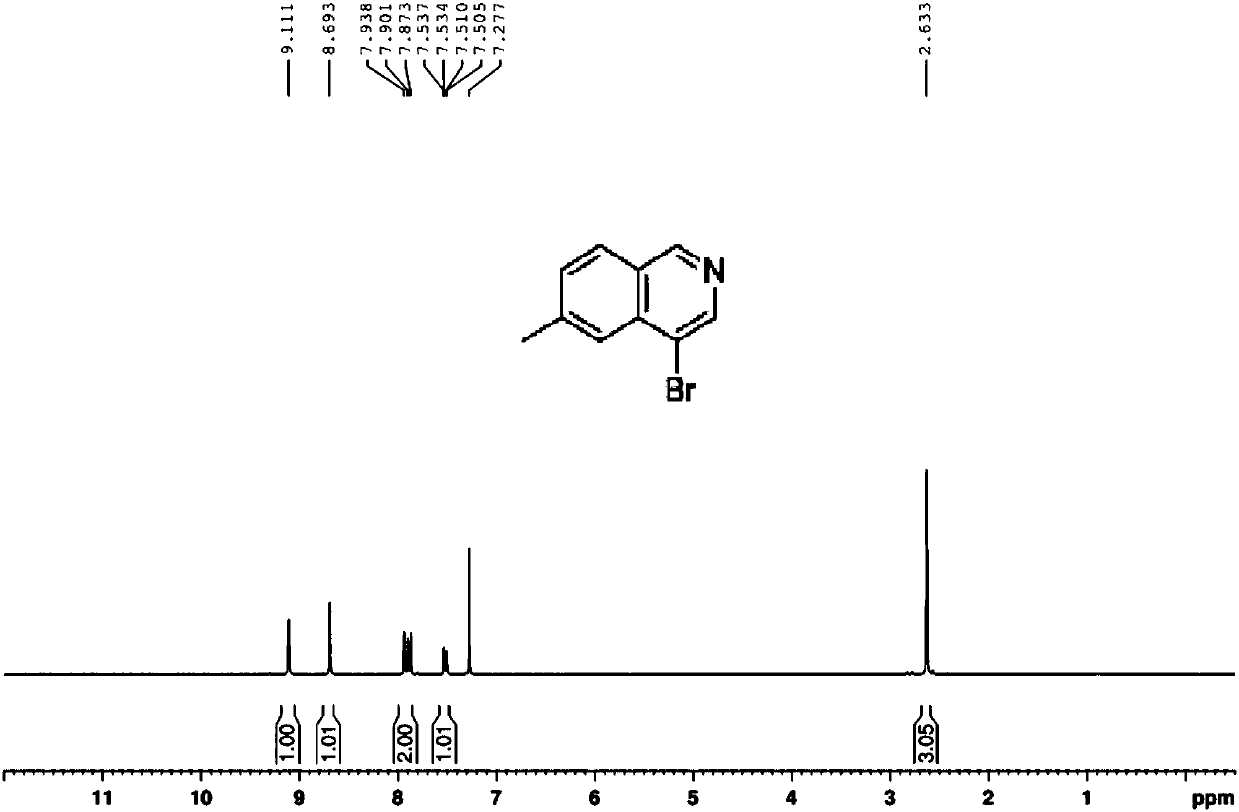
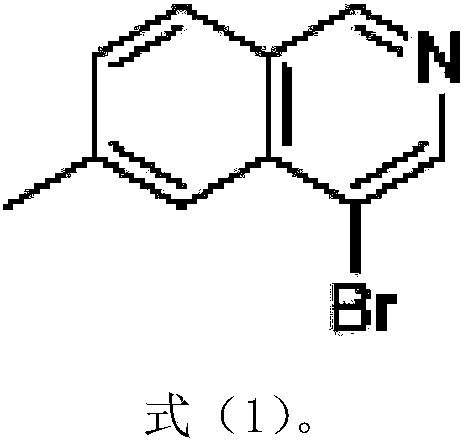
Preparation method of 6-methyl-4-bromo-isoquinoline
A technology of isoquinoline and methyl, which is applied in the field of pharmaceutical intermediates, can solve problems such as inability to promote, and achieve the effects of saving synthesis steps, saving raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] S1. Raw material preparation: 90 grams of amino acetal dimethyl acetal, 0.8 L of toluene and 100 grams of p-toluene, select a 2 L four-necked bottle with a water separator, first put 0.8 L of toluene, and add it under stirring Amino acetal dimethyl acetal 90 grams, after being completely dissolved by naked eyes, then add 100 grams of p-tolualdehyde, then increase the temperature, use the condenser tube to reflux and separate water for 3 hours, until no water is separated, end step S1 ;
[0023] S2. Distill the mixture in the four-necked flask obtained in S1 to remove most of the toluene, then add 50ml of toluene, and repeat the rotary distillation twice to obtain the crude product of step S2, 2,2-methyl-N-(4-methoxy phenylalkenyl) ethylamine;
[0024] S3. Take another four-necked bottle, put 0.8L tetrahydrofuran in it and stir in the four-necked bottle, take 160 grams of the crude product of step S2 2,2-methyl-N-(4-methoxyphenylenyl)ethyl Add the amine into the 0.8L t...
Embodiment 2
[0030] S1. Raw material preparation: 102 grams of amino acetal dimethyl acetal, 1 L of toluene and 105 grams of p-toluene, select a 2 L four-necked bottle with a water separator, first put 1 L of toluene, and add amino ethyl under stirring 102 grams of acetal dimethyl acetal, after being completely dissolved by naked eyes, then add 105 grams of p-tolualdehyde, then increase the temperature, use the condenser to reflux and separate water for 3 hours, until no water is separated, and step S1 is ended;
[0031] S2. Distill the mixture in the four-neck flask obtained in S1, remove most of the toluene, then add 100ml of toluene, and repeat the rotary steaming 3 times to obtain the crude product 2,2-methyl-N-(4-methoxy phenylalkenyl) ethylamine;
[0032] S3. Take another four-necked bottle, put 1L tetrahydrofuran inside and stir in the four-necked bottle, take 185 grams of the crude product of step S2, 2,2-methyl-N-(4-methoxyphenylenyl)ethylamine , into the 1L tetrahydrofuran, and ...
Embodiment 3
[0038] S1. Raw material preparation: 110 grams of amino acetal dimethyl acetal, 1.2 L of toluene and 110 grams of p-toluene aldehyde, select a 2 L four-necked bottle with a water separator, first put 1.2 L of toluene, and add it under stirring Amino acetal dimethyl acetal 110 grams, after being completely dissolved by naked eyes, then add 110 grams of p-tolualdehyde, then increase the temperature, use the condenser tube to reflux and separate water for 3 hours, until no water is separated, end step S1 ;
[0039] S2. Distill the mixture in the four-necked flask obtained in S1 to remove most of the toluene, then add 150ml of toluene, and repeat the rotary steaming 4 times to obtain the crude product 2,2-methyl-N-(4-methoxy phenylalkenyl) ethylamine;
[0040] S3. Take another four-necked bottle, put 1.2L tetrahydrofuran inside and stir in the four-necked bottle, take 200 grams of the crude product of step S2 2,2-methyl-N-(4-methoxyphenylenyl)ethyl Add the amine into the 1.2L te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com