Synthetic method of alpha,alpha,alpha-trifluoro-p-tolualdehyde
A technology of trifluoromethylbenzaldehyde and its synthesis method, which is applied in the direction of chemical instruments and methods, preparation of carbonyl compounds by hydrolysis, organic compound/hydride/coordination complex catalyst, etc., and can solve the problems of harsh reaction conditions, difficult industrial production, Deal with problems such as high pressure to achieve catalyst stability, high reactivity and selectivity, and stable performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation condition of table 1 catalyst
[0045]
[0046]
[0047] The embodiment of the present invention uses gas chromatography to detect anisole, and the conditions are as follows:
[0048] GC Agilent 6890 equivalent instrument
[0049] FID detector, chromatography workstation
[0050] ZB-5 (30m×0.32mm×0.25μm) equivalent chromatographic column
[0051] Hydrogen flow rate: 30ml / min
[0052] Air flow rate: 300ml / min
[0053] Carrier gas (high purity nitrogen) flow rate: 1.0ml / min
[0054] Gasification chamber temperature: 260°C
[0055] Detector temperature: 260°C
[0056] Central control conditions:
[0057] Initial column temperature: 120°C
[0058] End column temperature: 260°C
[0059] Heating rate: 20°C / min
[0060] Product Conditions:
[0061] Column temperature: run at 100°C for 20min
Embodiment 1
[0062] The preparation of embodiment 1 p-trifluoromethylbenzaldehyde
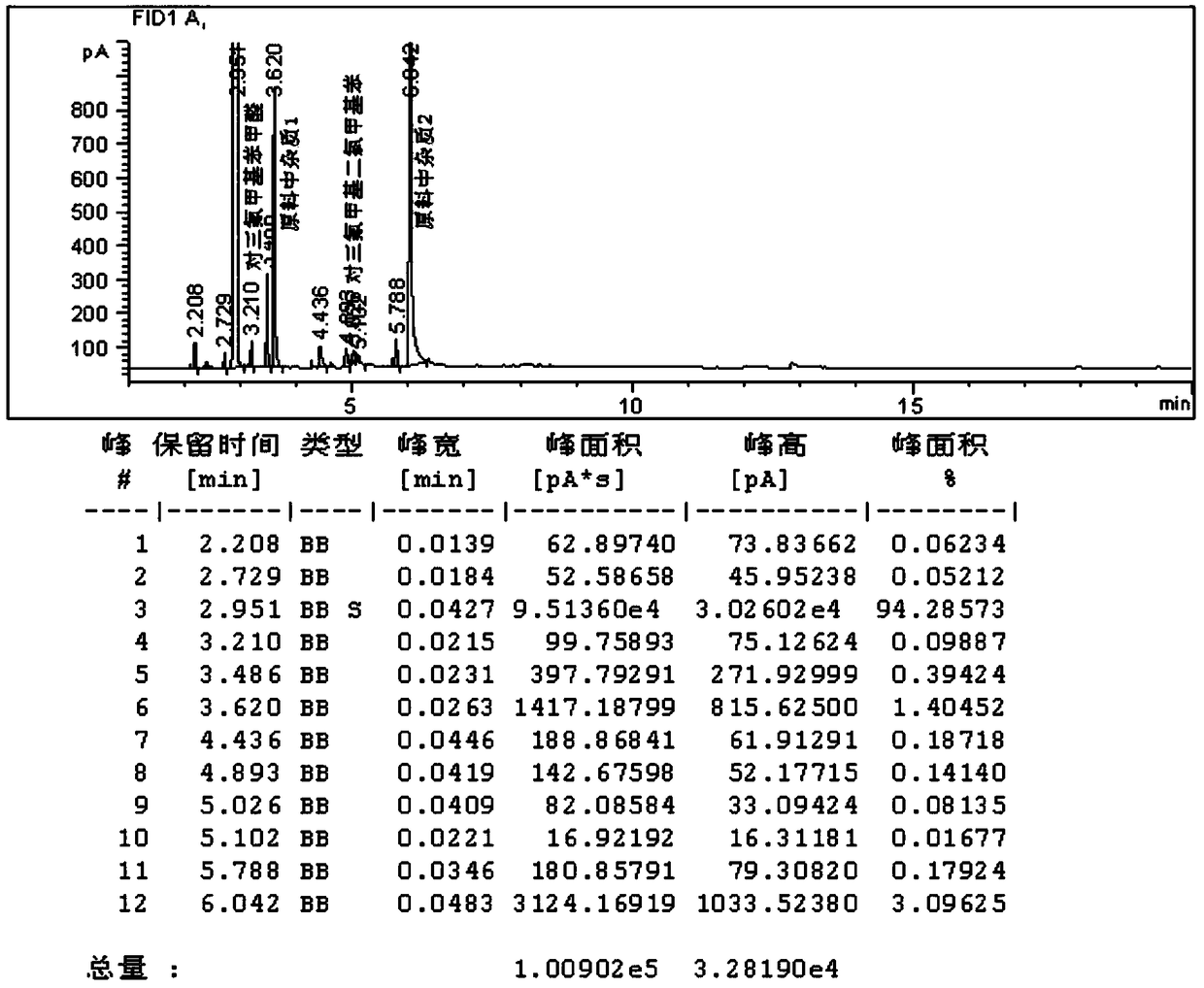
[0063] ①Hydrolysis reaction: Put 250g (1mol) of industrial crude product p-trifluoromethyldichloromethylbenzene and catalyst No. 12.5g, stir and heat up to 110-120°C, at this temperature, slowly drop 50g deionized water into the reaction system through the dropping funnel, dropwise for 2.5h, after the dropwise addition, keep warm for 1h, take a sample from the kettle to test the three The remaining 0.14% of fluoromethyl dichloromethylbenzene (see the gas chromatogram for details figure 2 ), the reaction ends.
[0064] ②Post-treatment: ①After the reaction, the temperature of the kettle was lowered to 90°C, 10g of deionized water was added, and allowed to stand still for 1 hour, 55g of the water layer containing the catalyst was separated, and the organic phase was washed twice with 50g of deionized water to obtain the The crude product of tolualdehyde was 188g. After the washing water was combined with th...
Embodiment 2
[0066] The preparation of embodiment 2 p-trifluoromethylbenzaldehyde
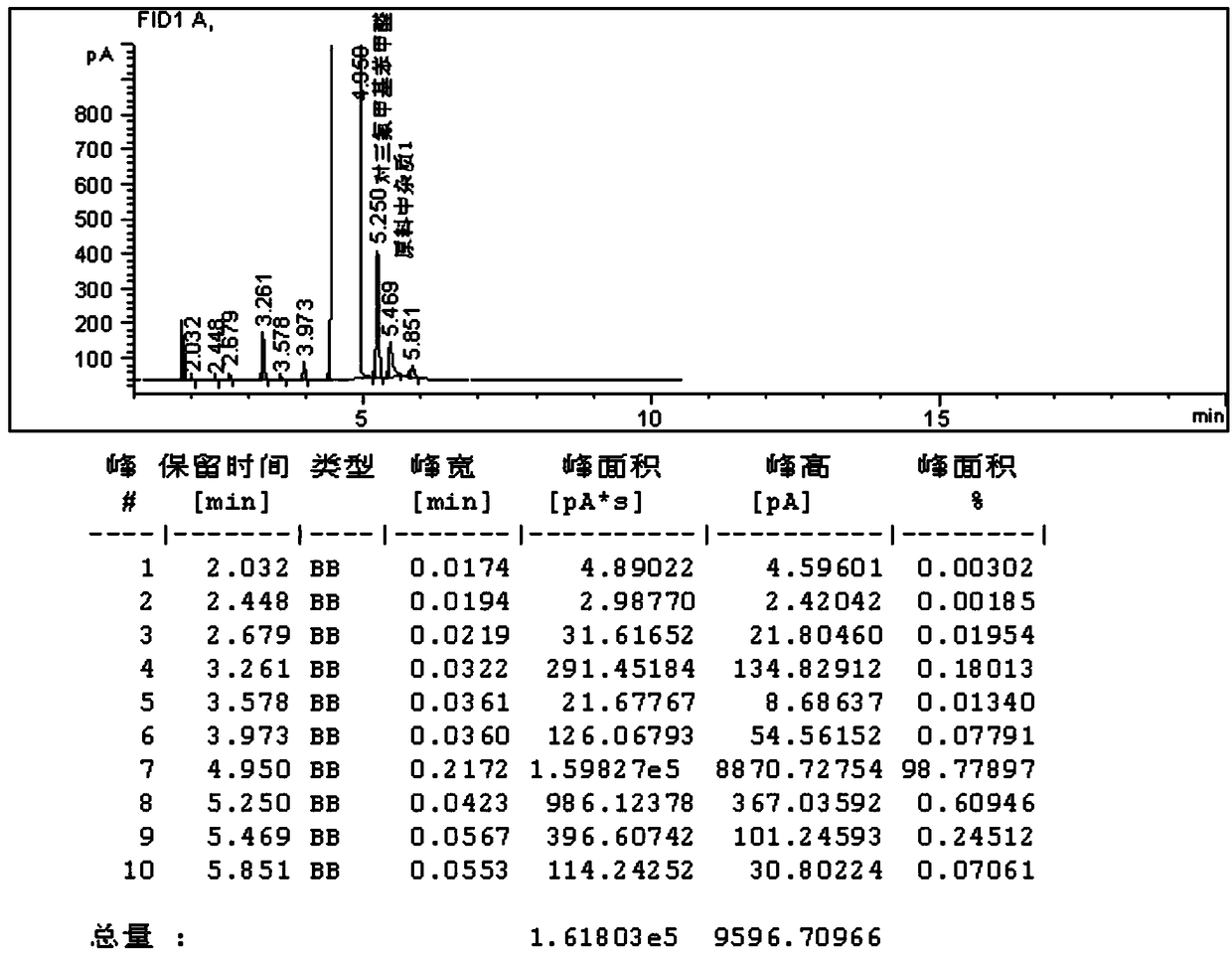
[0067] The reaction process is the same as Example 1, and the difference from Example 1 is that in Example 1, No. 1 catalyst is replaced with No. 2 catalyst 25g, and 25g of deionized water is used. Others are unchanged, and 26g of catalyst is reclaimed after the reaction to obtain 99.2 % p-trifluoromethylbenzaldehyde 167g, the product yield is 95.1%, and the distillation residue is 2.9g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com