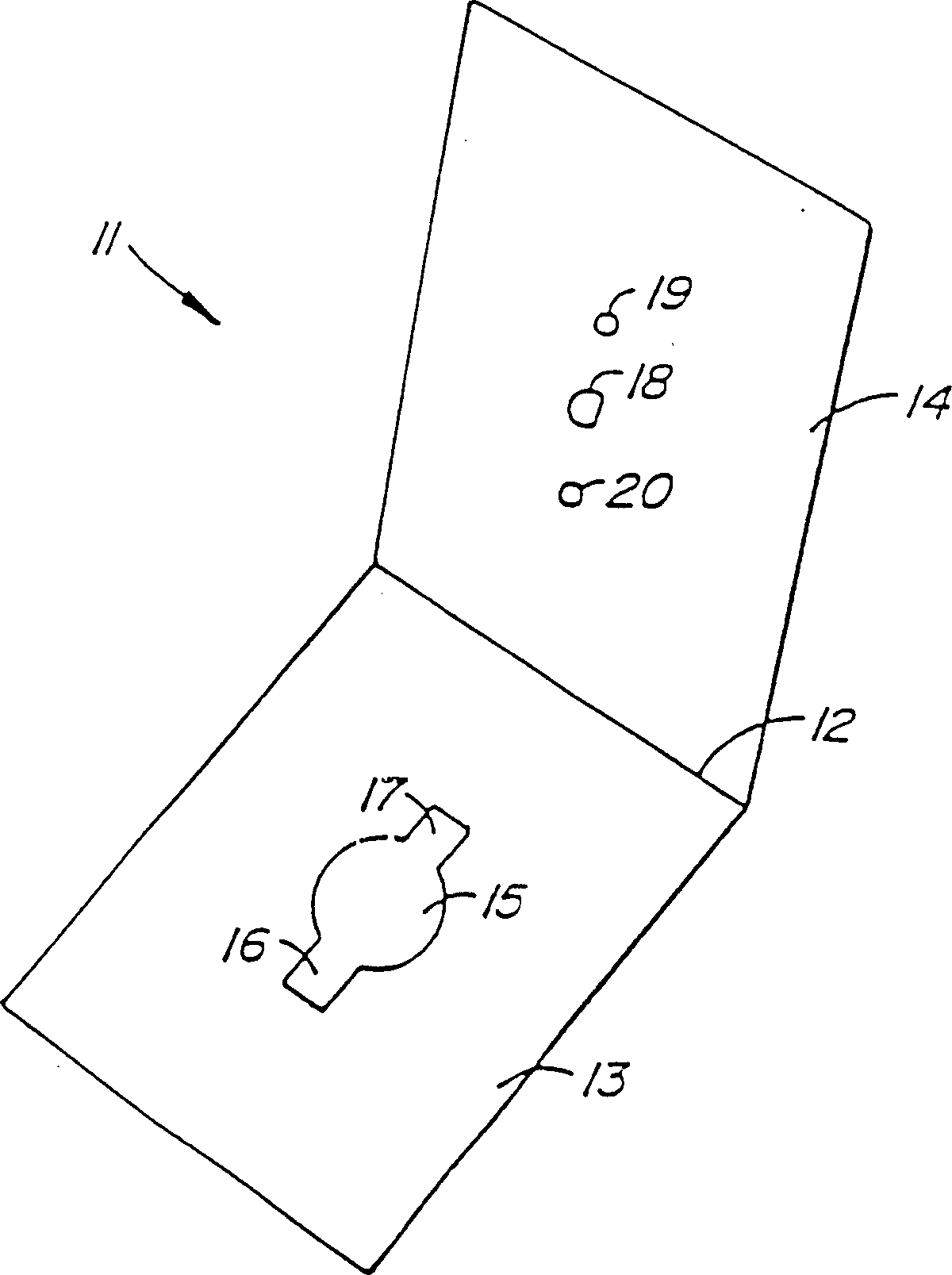
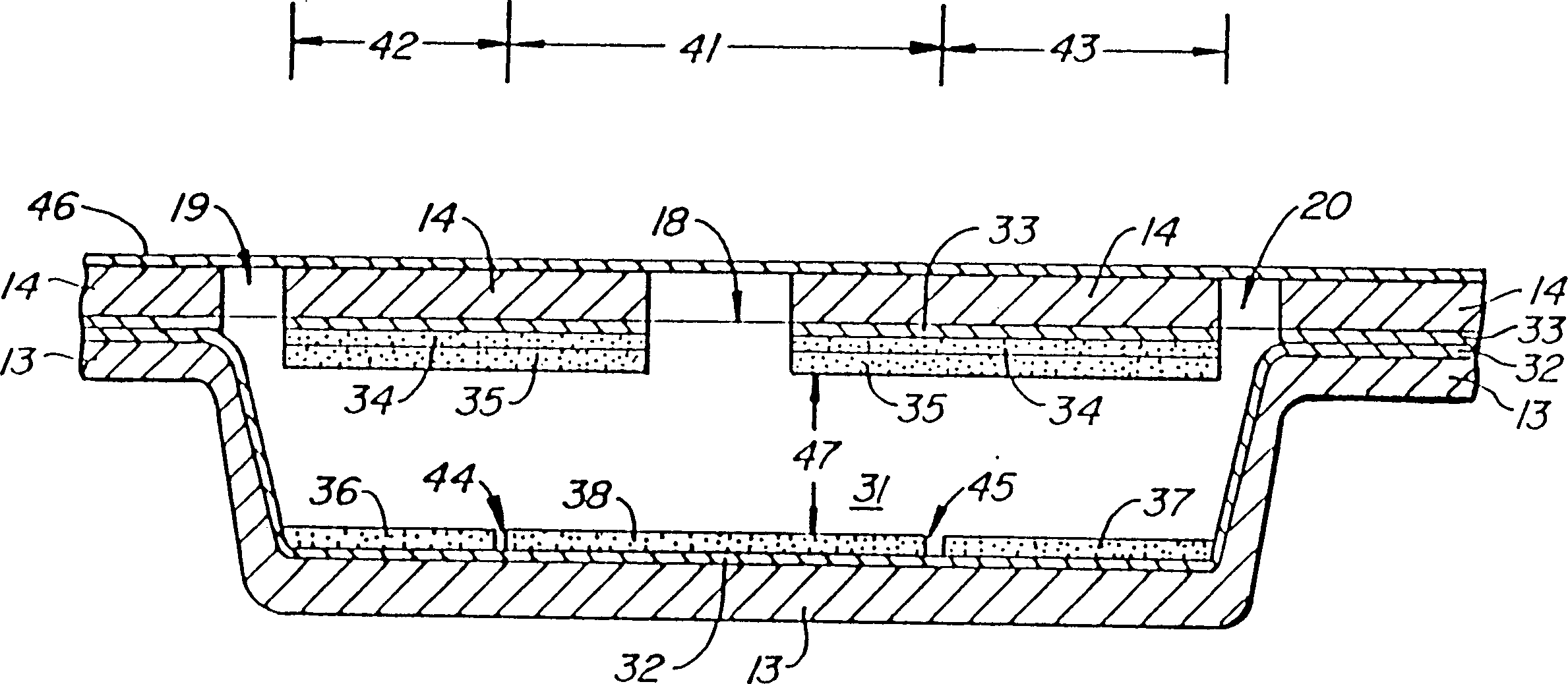
Reporter enzyme release tech, method of assaying for the presence of aspartic proteases and other hydrolytic enzyme activities
A technology of aspartic acid and hydrolase, which is applied in the direction of analytical materials, biological material analysis, biochemical equipment and methods, etc., can solve the problems of not developing aspartic acid protease, time-consuming and labor-intensive, etc., to save money and use, Ease of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0137] I. Preparation
[0138] A. Preparation of Cyanogen Bromide-Activated Solid Support
[0139] 1. Materials
[0140] a. Solid insoluble carrier (Sepharose 4B (Sepharose 4B), and Sigmacell
[0141] 20 [purchased from Sigma Chemical Co.])
[0142] b. Distilled water and ice made from distilled water
[0143] c.4.0M NaOH
[0144] d. Solid CNBr
[0145] e. Coupling buffer (0.1M NaHCO containing 0.5M NaCl 3 )
[0146] f. Magnetic stirring motor and stirring rod; pH meter; chemical fume hood
[0147] 2. Steps
[0148]Add 10 milliliters (10 ml) of cooled distilled water to 5 g of wet, washed solid carrier, and cool the mixture down to 10°C to 15°C. The pH of the suspension was adjusted to 10.8 with 4.0 M NaOH, and crushed solid CNBr was added at a rate of 100 mg per gram of moist solid carrier. Add 4.0M NaOH as needed to maintain pH 10.8, and allow the temperature of the suspension to rise to 18°C to 20°C during the activation process. Activation was considered co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com