Application of oligomeric stilbene compounds
A stilbene compound and compound technology are applied in the field of medical use of oligomeric stilbene compounds, and can solve problems such as side effects of patients and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
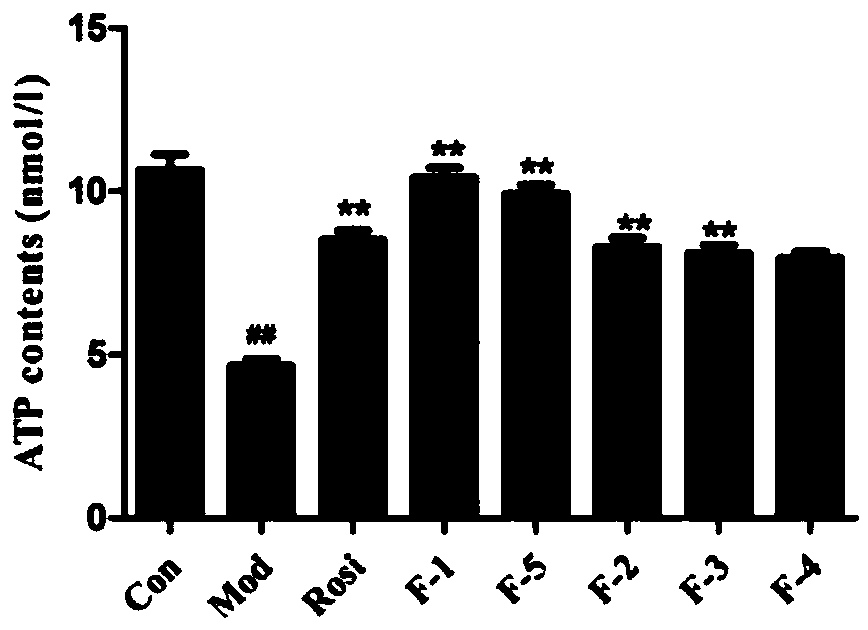
[0076] The application of the present invention will be further described through specific examples and experiments below, and the effect of proanthocyanidins on inhibiting cell differentiation will be verified and explained.
[0077] Experimental materials and experimental preparation
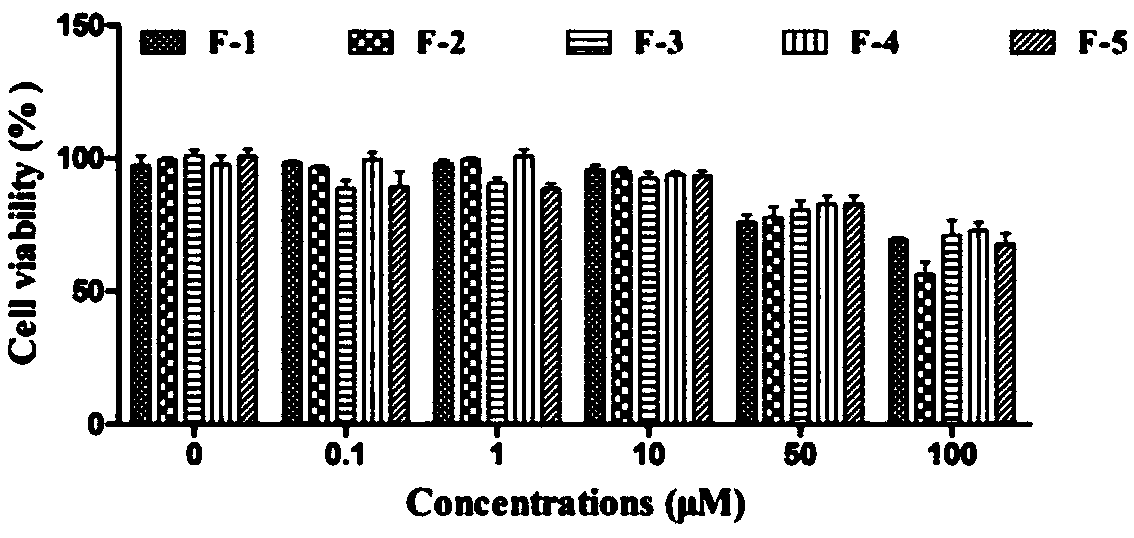
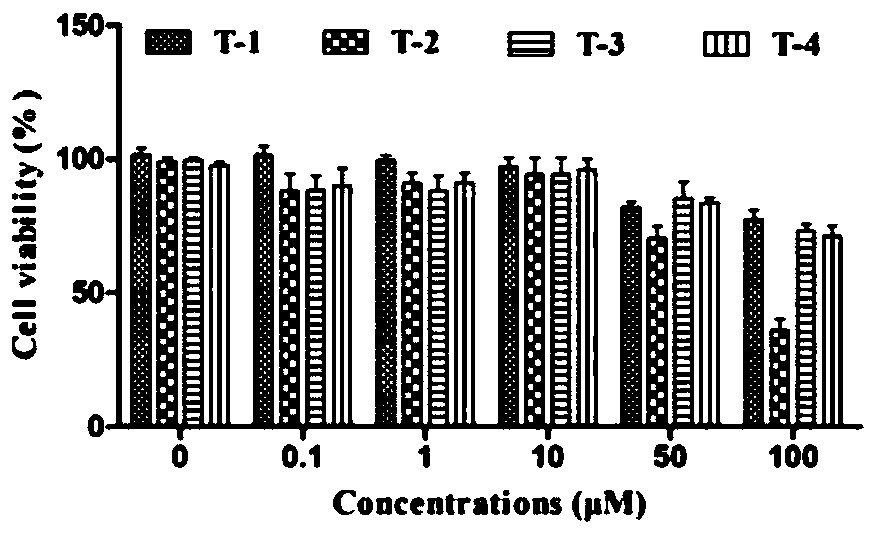
[0078] Test samples: including 9 oligostilbene compounds: Vitisin A(F-1), Vitisin B(F-2), VitisinC(F-3), Vitisin D(F-4), Cis-vitisinA(F -5), Resveratrol (T-1), ε-viniferin (T-2), Ampelopsin B (T-3), Hydroxyvitisin D (T-4), are all isolated and purified from the seeds of Sinus cuspidatum, and tested by HPLC The detection purity is above 95%, and its chemical structural formula is as follows:
[0079]
[0080]
[0081] Experimental method: the present invention uses statistical software SPSS 17.0 to carry out the analysis and arrangement of experimental data, and statistical results use mean ± standard value Indicates that the comparison of the means between the two groups adopts the in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com