Application of puerarin in V crystal form in preparing drug for preventing and/or treating liver injuries caused by diabetes mellitus
A technology of puerarin and diabetes, which is applied in the field of medicine, can solve the problems of inaccurate curative effect and slow onset, and achieve the effect of alleviating fatty liver and lowering blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Embodiment 1, the effect of crystal V-type puerarin on fructosamine in type 1 diabetic mice
[0048] Experimental method: 100 mice were adaptively fed for 1 week, and 12 were randomly selected as the normal control group (NC), and the rest were used as the diabetes model group. All animals were fasted overnight for 12 hours, and the mice in the model group were intraperitoneally injected with 130 mg·kg at a volume of 0.1 mL / 10 g -1 Streptozocin (STZ) (prepared with pH 4.4 citric acid buffer solution and placed on ice before use), and the mice in the normal control group were only injected with citric acid buffer solution. After 72 hours of STZ injection, the mice were fasted for 4 hours, blood was taken from the tip of the tail, and the fasting blood glucose was measured. Select fasting blood glucose greater than 16.7mmol L -1 The animal is a mouse model of type 1 diabetes. The diabetic mice successfully modeled were randomly divided into the following groups: model ...
Embodiment 2
[0055] Embodiment 2, the effect of crystal V-type puerarin on the glucose tolerance of type 1 diabetic mice
[0056] Experimental method: On the 19th day of administration, an oral glucose tolerance test (OGTT) was performed. Animals were fasted for 4 hours, blood was taken from the inner canthus of the eyes, and glucose was measured by hexokinase method as the blood glucose level at 0 min before sugar administration. Then give 2.5g·kg by gavage according to the volume of 0.1mL / 10g -1 Glucose solution, blood was collected from the inner canthus of the eyes at 30 minutes, 60 minutes and 120 minutes after the glucose was fed, and the blood glucose levels at each time point were measured. Draw the glucose tolerance curve and calculate the area under the curve (AUC) of blood glucose-time, where AUC = 0.25×FBG 0h+0.5×FBG 0.5h+0.75×FBG 1h+0.5×FBG 2h, where FBG 0h means 0h fasting blood glucose levels.
[0057] Experimental results:
[0058] As shown in Table 2, each dose group o...
Embodiment 3
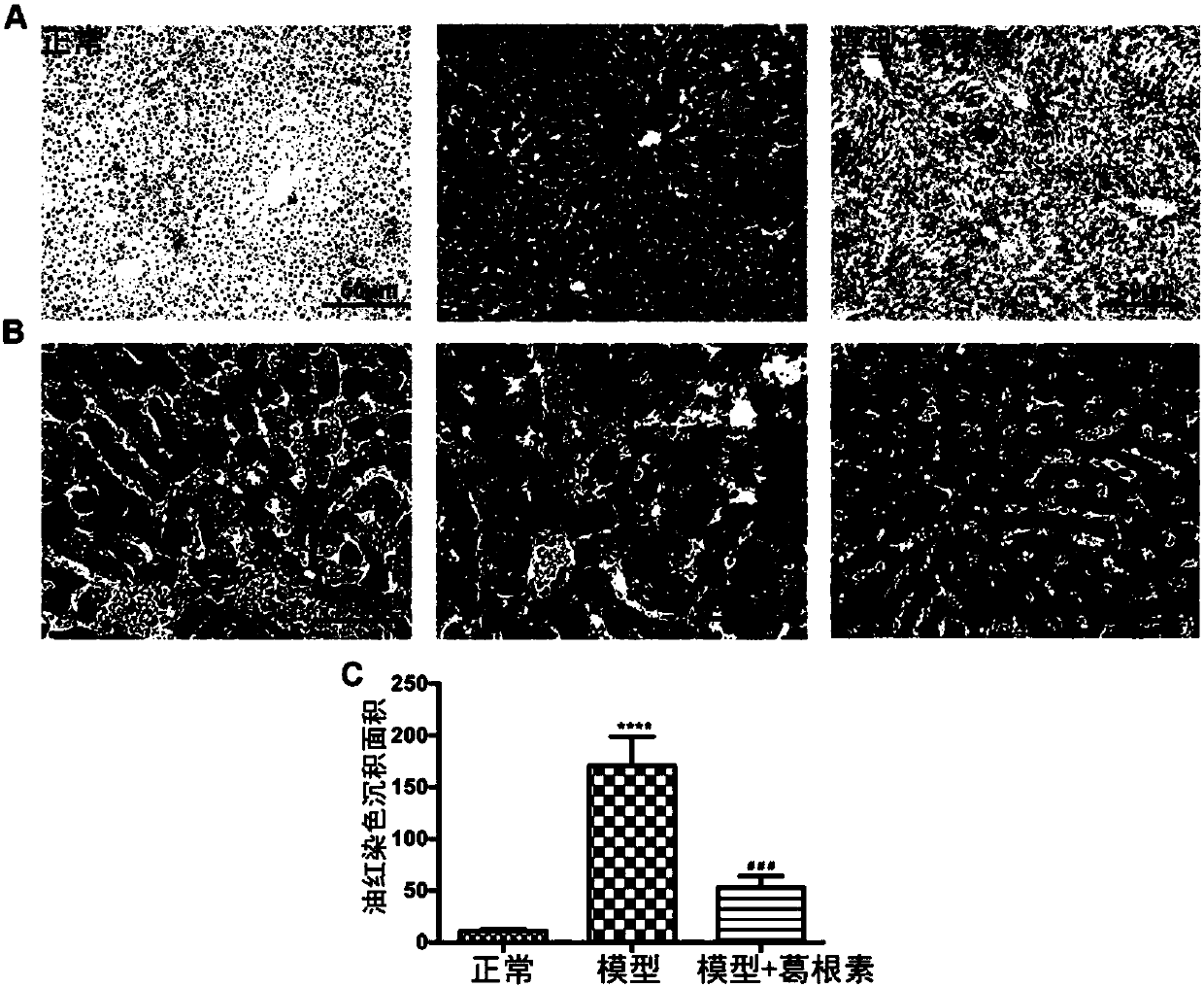
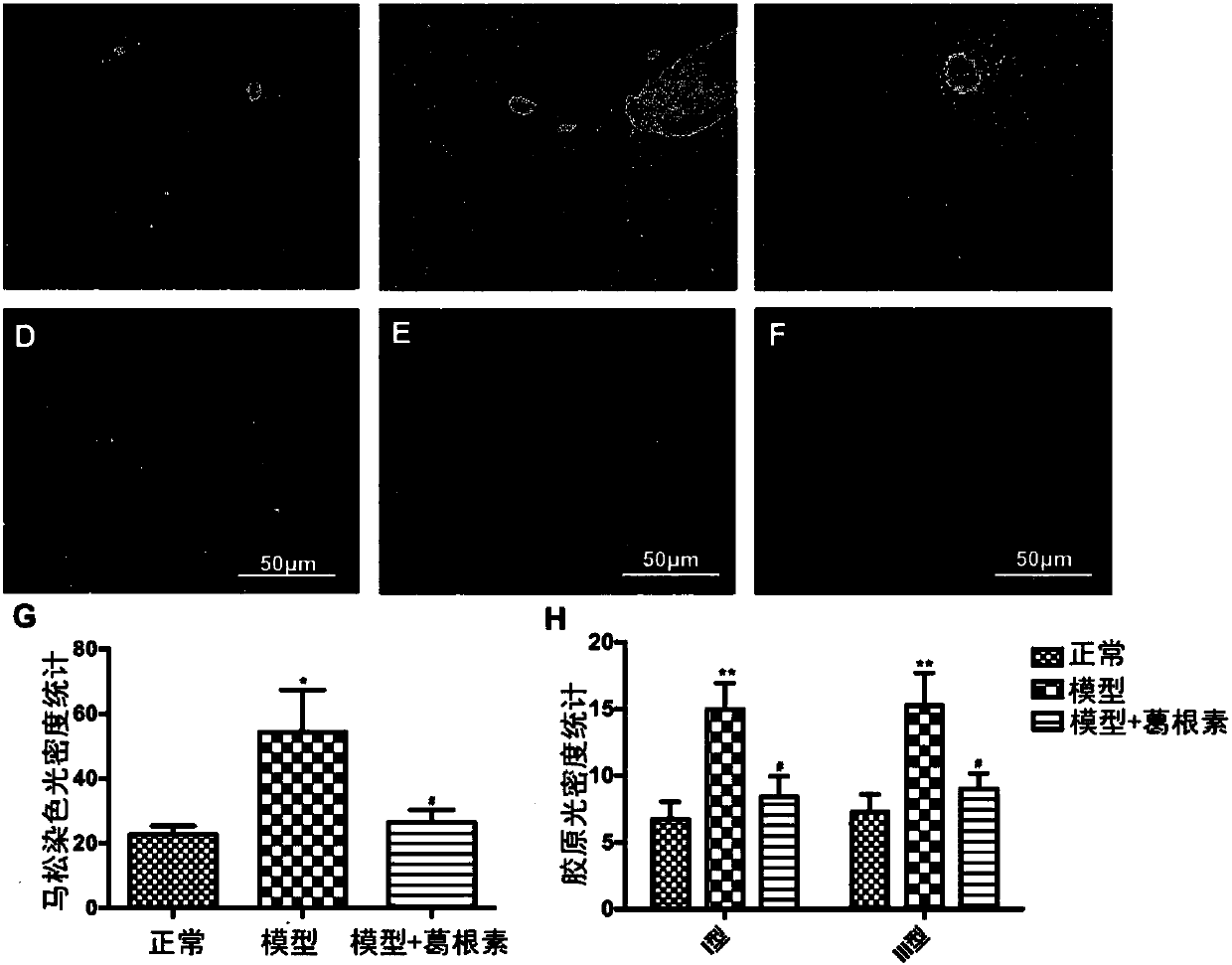
[0062] Embodiment 3, the effect of crystal V puerarin on the liver function of type 1 diabetic mice
[0063] experimental method:
[0064] At the end of the experiment, the animals were weighed, the eyeballs were removed to take blood, anticoagulated with heparin, centrifuged at 5000r for 15min at 4°C, and the supernatant was taken. An automatic biochemical analyzer was used to detect the levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
[0065] Experimental results:
[0066] After 3 weeks of administration, ALT and AST (P<0.05, P<0.01) in crystal V puerarin low and middle dose groups decreased significantly, although ALT in high dose group tended to decrease, but the difference was not significant.
[0067] Table 3 Effect of crystal V puerarin on liver index and liver function in type 1 diabetic mice
[0068]
[0069] n=10~12, Mean±SD. * P** P# P## P<0.01 compared with model control group
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com