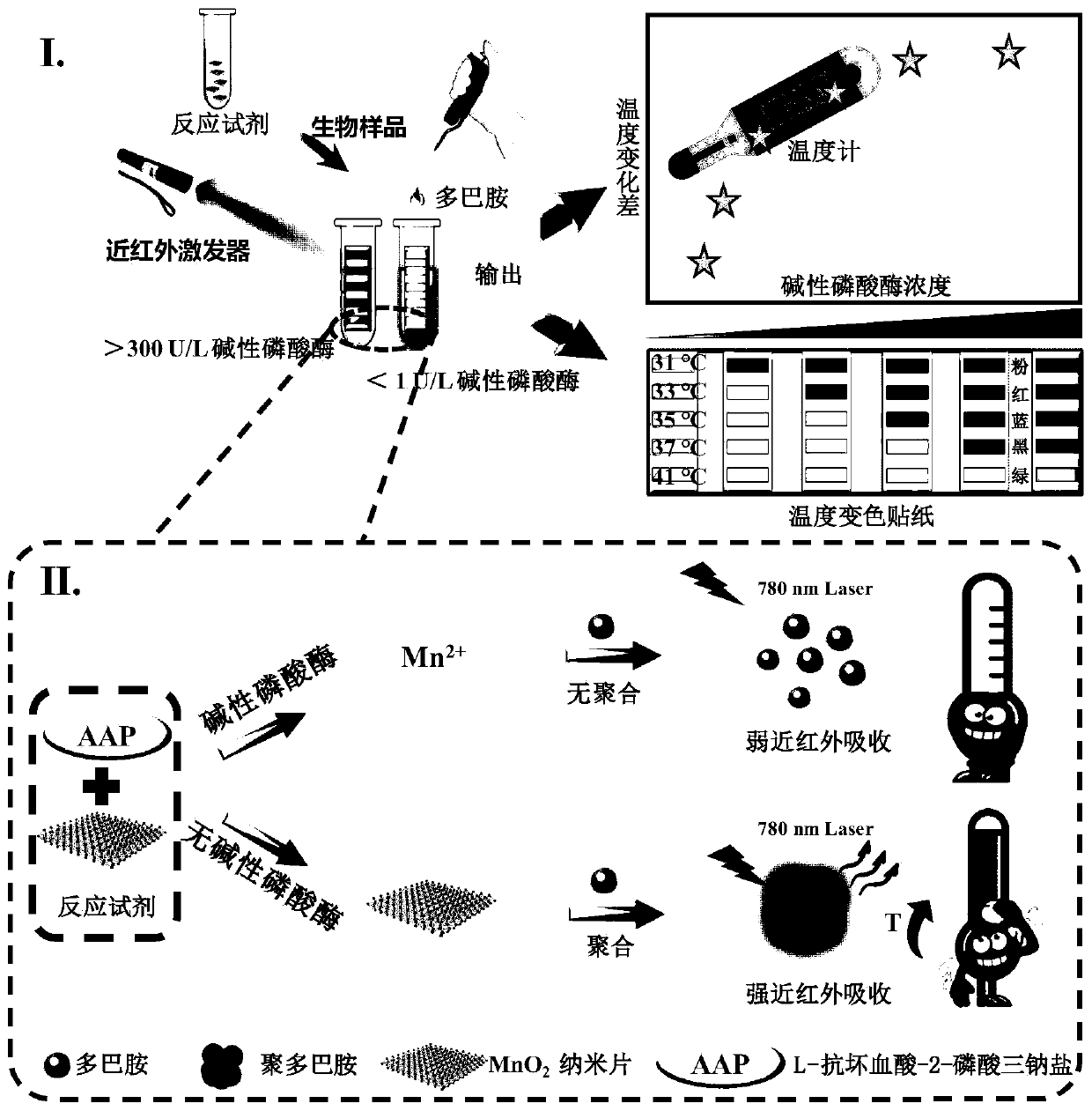
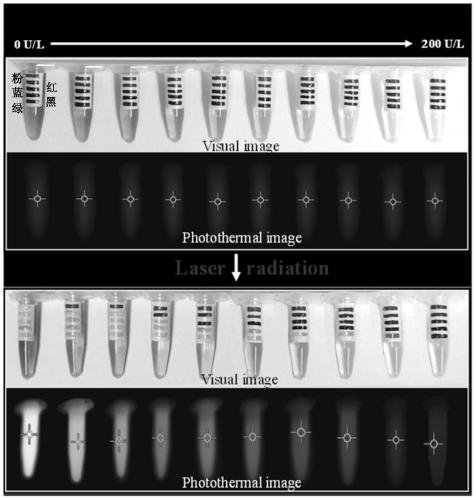
Portable detection kit for alkaline phosphatase based on temperature change and its application
A portable detection and detection reagent technology, applied in the field of biosensing, which can solve the problems of low sensitivity and complex surface modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A portable alkaline phosphatase detection kit based on temperature changes of the present invention, comprising: 20 μg / mL manganese dioxide nanosheets, 23 μg / mL L-ascorbic acid-2-phosphate trisodium salt, 0.48 mg / mL Magnesium chloride and 20 μg / mL dopamine.
[0047] An application of the alkaline phosphatase portable detection kit of the present embodiment in the detection of alkaline phosphatase, its application method comprises the following steps:
[0048] (1) Add manganese dioxide nanosheets and L-ascorbic acid-2-phosphate trisodium salt into Tris-HCl buffer solution ((the composition of Tris-HCl buffer solution is: 10mmol / L Tris-HCl buffer, pH 7.4) A detection reagent was obtained, the concentration of manganese dioxide nanosheets in the detection reagent was 20 μg / mL, and the concentration of L-ascorbic acid-2-phosphate trisodium salt was 23 μg / mL.
[0049] (2) Prepare a standard solution of alkaline phosphatase: use Tris-HCl buffer as a solvent, and prepare a co...
Embodiment 2
[0060] Investigate the influence of different buffer solutions on the detection effect:
[0061] Manganese dioxide nanosheets and L-ascorbic acid-2-phosphate trisodium salt were added to the phosphate buffer (the composition of the phosphate buffer is: 10mmol / L phosphate buffer, pH 7.4, the composition of the phosphate buffer is the same throughout the text) , Tris-HCl buffer (the composition of Tris-HCl buffer is: 10mmol / L Tris-HCl buffer, pH 7.4, the composition of the phosphate buffer is the same throughout the text), HEPES buffer (10mmol / L HEPES buffer, pH 7.4 , the composition of the HEPES buffer solution is the same throughout the text), and detected according to the method of Example 1.
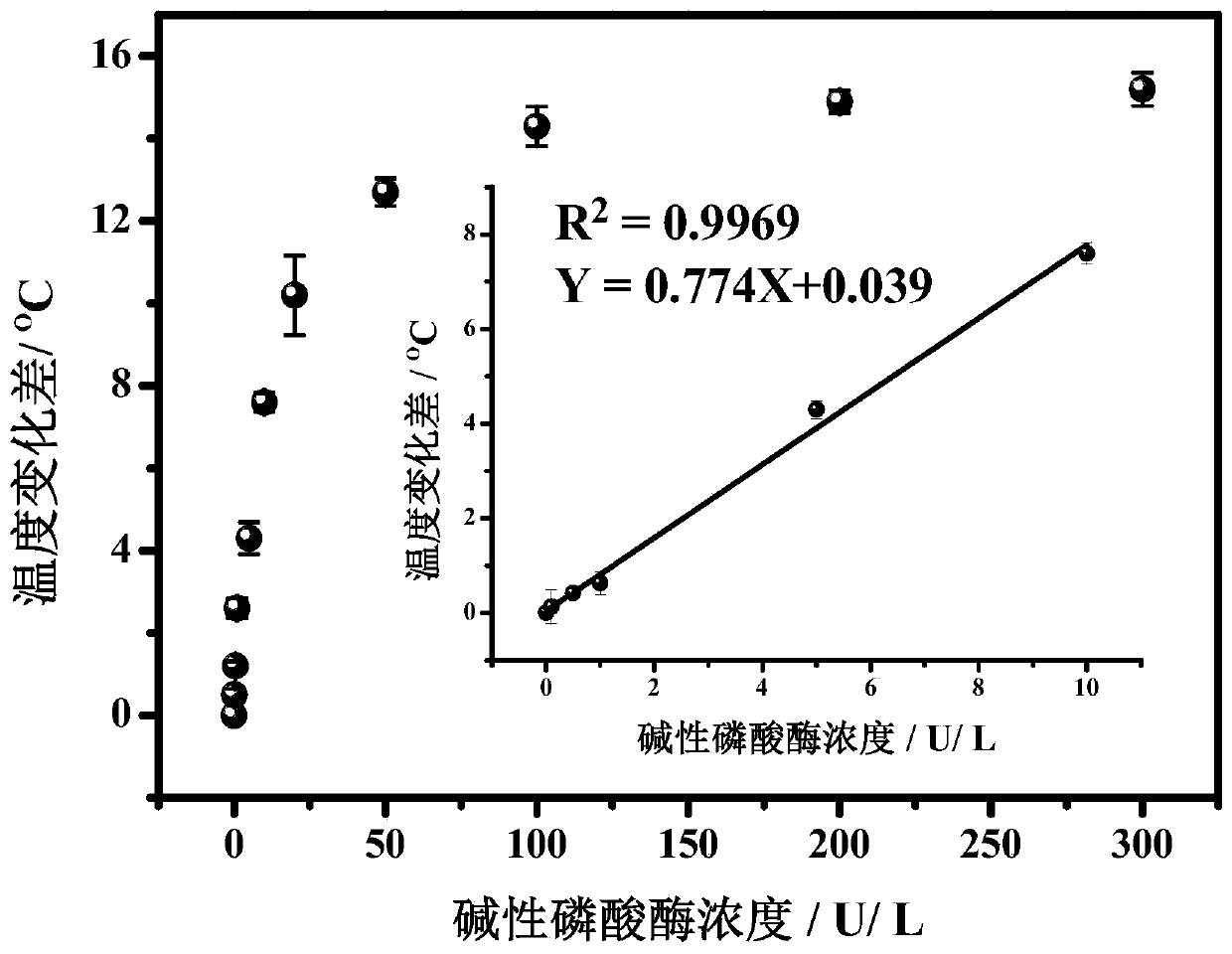
[0062] Test results such as Figure 4 Shown: The temperature change value is the largest and the signal is the strongest in Tris-HCl buffer. Therefore, Tris-HCl buffer is selected as the best buffer in subsequent examples.
Embodiment 3
[0064] Investigate the influence of dopamine solution concentration on the detection effect:
[0065] Dopamine was dissolved in Tris-HCl buffer, and different concentrations of dopamine solutions were prepared: 0 μg / mL, 5 μg / mL, 10 μg / mL, 15 μg / mL, 20 μg / mL, 30 μg / mL and 40 μg / mL. According to the steps of Example 1, different concentrations of dopamine solutions were added for detection.
[0066] Test results such as Figure 5 As shown: when the dopamine concentration in the detection reagent is 20-40 μg / mL, it reaches a plateau, the temperature change value is the largest, and the signal is the strongest. Therefore, 20 μg / mL is selected as the optimal concentration of dopamine solution in subsequent examples.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com