Magnetic highly-graphitized carbon-based photo-thermal composite phase change material and application thereof
A technology of composite phase change materials and graphitized carbon, which is applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of cumbersome preparation methods, single function of carbon materials, neglect of the adjustment and enhancement effects of metal materials, and achieve The effect of high degree of graphitization, improved crystallization ability, enhanced stability and light-to-heat conversion ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] (1) Preparation of cobalt MOF
[0052] Dissolve 3mmol of terephthalic acid and 3mmol of cobalt acetate tetrahydrate in 25ml of dimethyl sulfoxide, stir at room temperature for 12h, wash with N,N-dimethylformamide and ethanol three times as detergents, and place in a vacuum oven at 60°C Dry in medium for 24h.
[0053] (2) Preparation of magnetic graphitized carbon
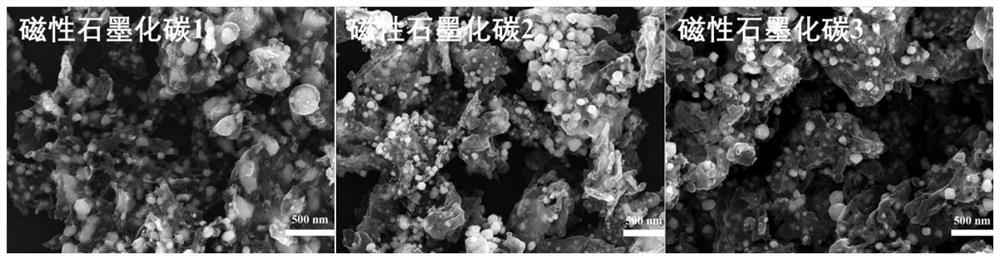
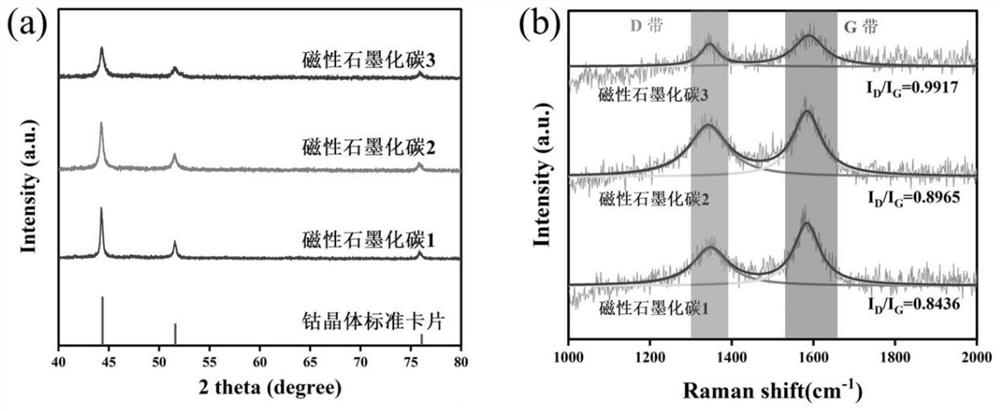
[0054] The MOF powder in step (1) was raised to 1000°C at a rate of 5°C / min in a nitrogen atmosphere in a tube furnace, and kept at 1000°C for 3 hours. The final product obtained was magnetic graphitized carbon 1.
[0055] (3) Preparation of magnetic graphitized carbon-based composite phase change materials
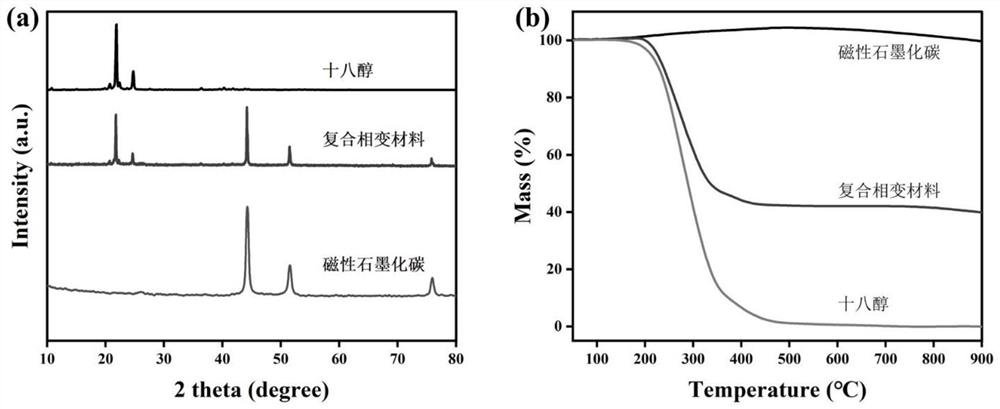
[0056] Dissolve 0.6g stearyl alcohol and 0.4g magnetic graphitized carbon in step (2) in 15ml ethanol, sonicate for 20min to dissolve stearyl alcohol, and mix evenly. Completely volatilized, the obtained solid is the composite phase change material 1.
Embodiment 2
[0058] (1) Preparation of cobalt MOF
[0059] Dissolve 3mmol terephthalic acid and 2mmol cobalt acetate tetrahydrate in 25ml dimethyl sulfoxide, stir at room temperature for 12h, wash three times with N,N-dimethylformamide and ethanol respectively, and place in a vacuum oven at 60°C Dry in medium for 24h.
[0060] (2) Preparation of magnetic graphitized carbon
[0061] The MOF powder in step (1) was raised to 1000°C at a rate of 5°C / min in a nitrogen atmosphere in a tube furnace, and kept at 1000°C for 3 hours. The final product obtained was magnetic graphitized carbon 2.
[0062] (3) Preparation of magnetic graphitized carbon-based composite phase change materials
[0063] Dissolve 0.6g of stearyl alcohol and 0.4g of magnetic graphitized carbon in step (2) in 15ml of ethanol, sonicate for 20min to dissolve the stearyl alcohol, and mix evenly. Completely volatilized, the obtained solid is the composite phase change material 2.
[0064] The loading capacity of the phase cha...
Embodiment 3
[0066] (1) Preparation of cobalt MOF
[0067] Dissolve 3mmol terephthalic acid and 1mmol cobalt acetate tetrahydrate in 25ml dimethyl sulfoxide, stir at room temperature for 12h, wash with N,N-dimethylformamide and ethanol three times as detergents, and place in a vacuum oven at 60°C Dry in medium for 24h.
[0068] (2) Preparation of magnetic graphitized carbon
[0069] The MOF powder in step (1) was raised to 1000°C at a rate of 5°C / min in a nitrogen atmosphere in a tube furnace, and kept at 1000°C for 3 hours. The final product obtained was magnetic graphitized carbon 3.
[0070] (3) Preparation of magnetic graphitized carbon-based composite phase change materials
[0071] Dissolve 0.6g of stearyl alcohol and 0.4g of magnetic graphitized carbon in step (2) in 15ml of ethanol, sonicate for 20min to dissolve the stearyl alcohol, and mix evenly. Completely volatilize, the obtained solid is the composite phase change material 3.
[0072] The loading capacity of the phase cha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com