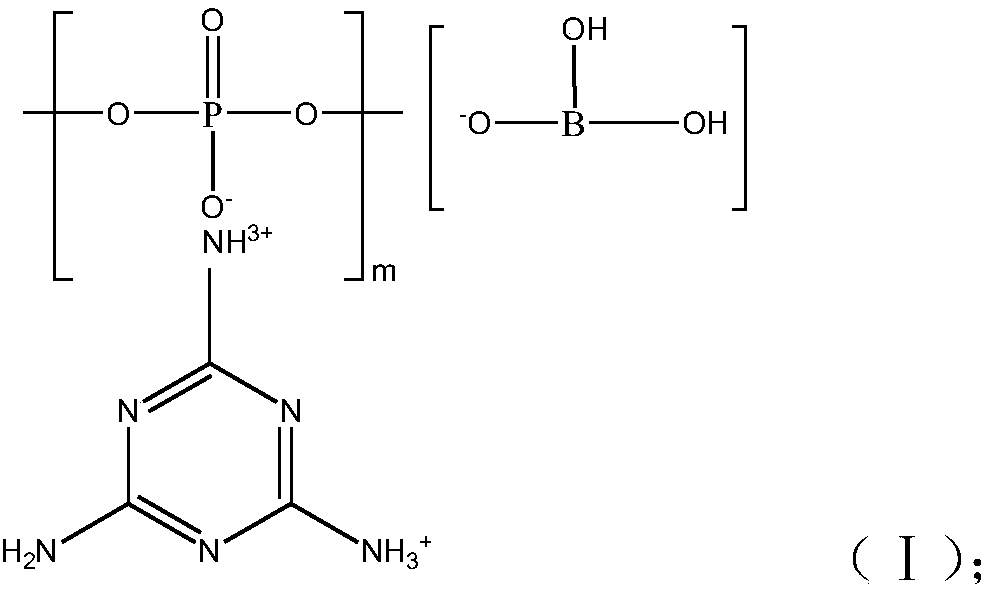
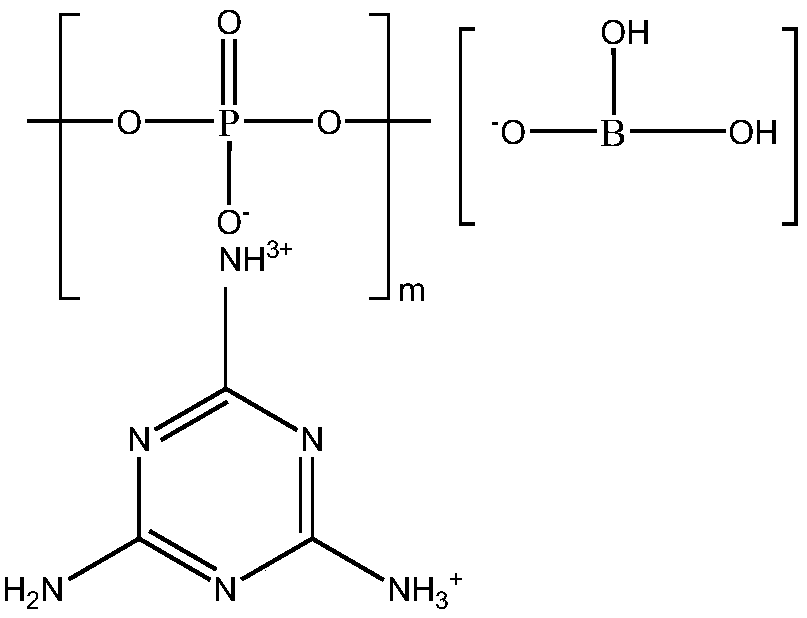
Halogen-free flame retardant melamine poly-phosphate borate as well as preparation method and application thereof
A technology of melamine polyphosphate borate and melamine polyphosphate, which is applied in the field of flame retardants, can solve the problems of low flame retardant efficiency, easy moisture absorption, and low carbon formation efficiency of melamine polyphosphate, and achieve high carbon formation efficiency and water absorption resistance Good performance, improved water absorption resistance and charcoal efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] a. Add 500mL of deionized water and 186g of melamine to a 1000mL four-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser, stir and heat to 80°C, slowly add 199g of 85wt% phosphoric acid dropwise, within about one hour After the dropwise addition, heat up and stir to 95°C, keep stirring for 2 hours, and increase the stirring speed when the viscosity becomes larger in the later stage. The suspension was filtered, washed several times with deionized water, and dried at 120° C. to obtain 323.7 g of an intermediate.
[0052] b. Add 1000mL of deionized water to a 2000mL four-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser, add 27.2g of boric acid, heat up to 80°C to completely dissolve the boric acid, and add 323.7g of the intermediate obtained in step a , keep the temperature at 80°C, and stir the reaction for 3 hours. The suspension was cooled, filtered and washed several times with deionized water, the...
Embodiment 2
[0073] a. Add 500mL of deionized water and 186g of melamine to a 1000mL four-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser, stir and heat to 60°C, then slowly add 154g of 85% phosphoric acid dropwise for about one hour After the internal dropwise addition, heat up and stir to 85° C., and stir for 1 hour. The viscosity increases later, and the stirring speed is increased. The suspension was filtered, washed several times with deionized water, and then dried at 120° C. to obtain 289.9 g of an intermediate.
[0074] b. Add 800mL of deionized water to a 2000mL four-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser, add 31.6g of boric acid, heat up and stir to completely dissolve the boric acid, continue to heat up to 90°C, and add step a to obtain 289.9g of the intermediate was kept at 100°C, and the reaction was stirred for 3.5h. The suspension was cooled, filtered and washed several times with deionized w...
Embodiment 3
[0081] a. Add 500mL of deionized water and 186g of melamine to a 1000mL four-neck flask equipped with an electric stirrer, a thermometer and a reflux condenser, stir and heat to 100°C, then slowly add 199g of 85% phosphoric acid dropwise for about two hours After the internal dropwise addition, heat up and stir to 90°C for 2h, filter the obtained slurry, wash with deionized water several times, and then dry at 140°C to obtain 332.1g of an intermediate.
[0082] b. Add 1000mL of deionized water and 31.1g of boric acid to a 2000mL four-necked flask equipped with an electric stirrer, a thermometer and a reflux condenser, heat up and stir to completely dissolve the boric acid, continue to heat up to 100°C, and add the obtained in step a 332.1g of the intermediate was kept at a temperature of 92°C, and the reaction was stirred for 3h. The suspension was cooled, filtered, washed several times with deionized water, and dried at 140°C to yield 313.9 g of intermediate product.
[0083...
PUM
| Property | Measurement | Unit |
|---|---|---|
| solubility (mass) | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| solubility (mass) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com