Synergistic antifungal compositions and methods thereof
A composition, antifungal technology, applied in the field of antimicrobial agents and pharmaceutical sciences, can solve the problem of high recurrence rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0264] Example 1: Medium-chain fatty acid / esters (C-1 to C- 14) Exemplary synergistic antifungal combinations with multiple antifungal agents
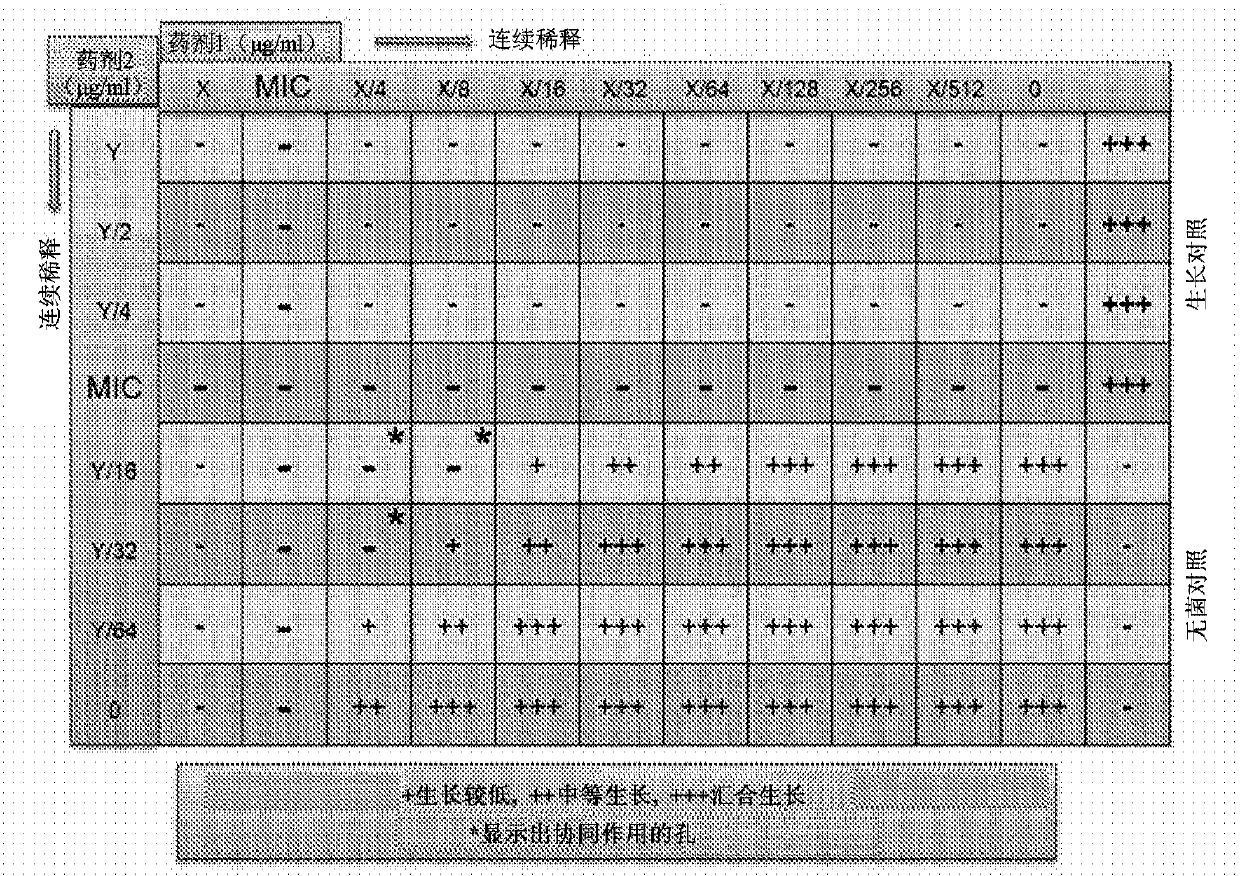
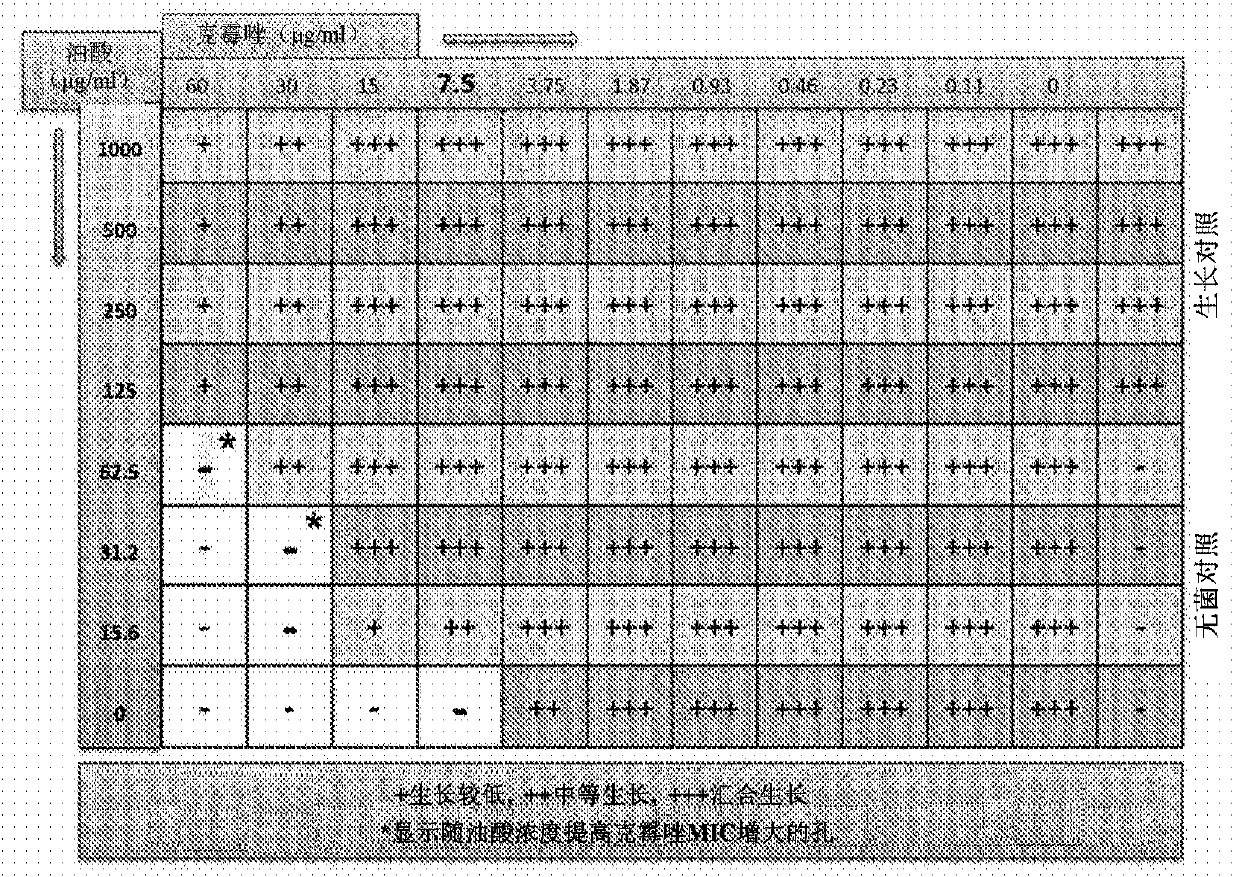
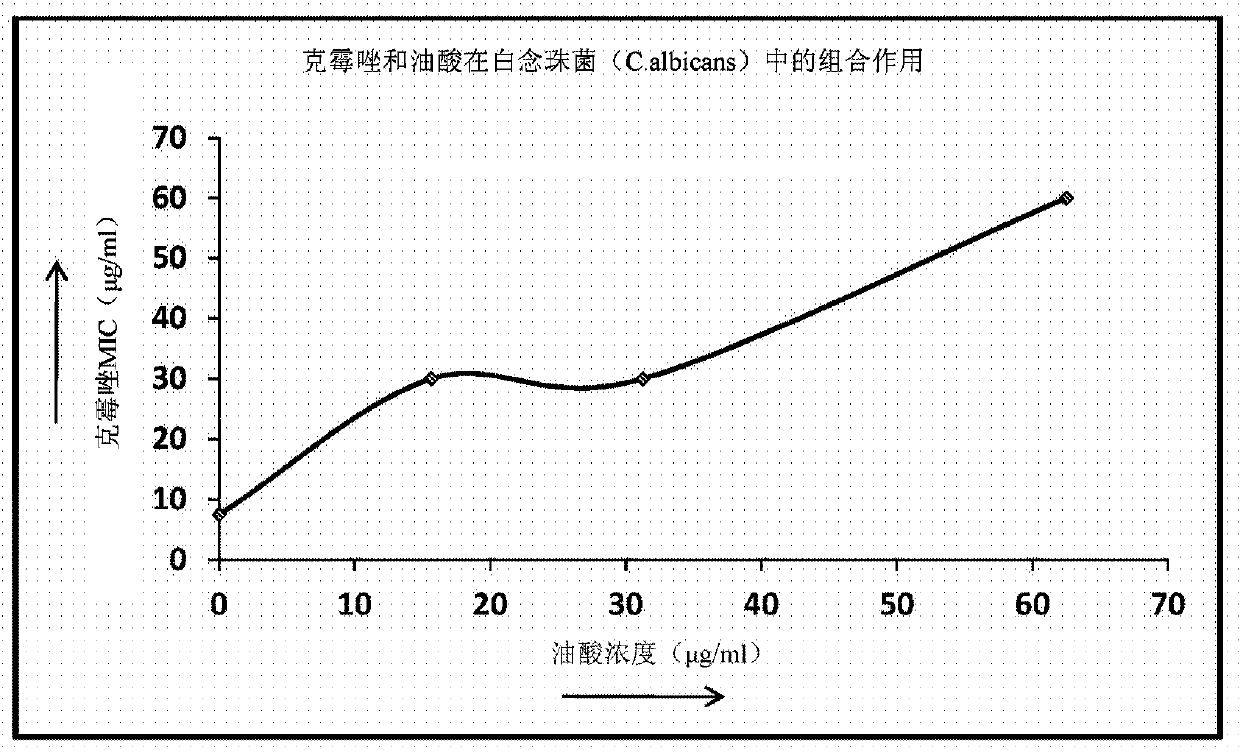
[0265] Using the above checkerboard layout protocol ( Figure 1A ), testing the ability of various medium chain fatty acids to enhance the activity of known antifungal agents. Concentrations of each test agent above and below its MIC were tested using serial dilutions. The same procedure was extended to test various medium chain fatty acids C-1 to C-14 (such as caprylic acid, undecylenic acid and lauric acid) against susceptible and resistant Trichophyton (filamentous fungus) and Candida (yeast). ) and esters thereof in combination with antifungal agents from various classes including azoles, allylamines, benzylamines, zinc pyrithione and piroctone olamine.
[0266] method : The enhancement of antifungal activity using ester derivatives of medium chain fatty acids was tested in a standard recognized in vitro assay system to dete...
Embodiment 2
[0305] Embodiment 2: the preparation of the various oil compositions containing piroctone olamine and octanoic acid
[0306] Compositions are prepared by dissolving the active agent in ethanol or isopropanol (IPA). Then, oleyl alcohol was added and stirred until a homogeneous solution was obtained. In addition to liquid paraffin, other excipients or additives are added and stirred to obtain a clear solution. Finally, make up to weight with liquid paraffin and stir until a homogeneous solution is obtained. The final formulation is a clear transparent oil solution. "Table 15" describes clear antifungal oil compositions containing piroctone olamine and medium chain fatty acids and / or esters as antifungal agents using various excipients or additives.
[0307] Table 15: Piroctone olamine-caprylic acid-oil composition
[0308]
[0309]
[0310] C-clear, ST-slightly cloudy, PO-piroctone olamine, IPA-isopropanol, OA-oleyl alcohol, Cap.A-caprylic acid, Toco.Ace-tocopheryl...
Embodiment 3
[0314] Embodiment 3: the preparation of the various oil compositions containing ketoconazole and octanoic acid
[0315] Compositions are prepared by dissolving the active agent in ethanol. Then, oleyl alcohol was added and stirred until a homogeneous solution was obtained. In addition to liquid paraffin, other excipients or additives are added and stirred to obtain a clear solution. Finally, make up to weight with liquid paraffin and stir until a homogeneous solution is obtained. The final formulation is a clear transparent oil solution. "Table 16" describes clear antifungal oil compositions containing ketoconazole and medium chain fatty acids and / or esters as antifungal agents using various excipients or additives.
[0316] Table 16: Ketoconazole-octanoic acid-oil compositions
[0317]
[0318] C-clear and transparent, ST-slightly turbid
[0319] result:
[0320] 1. Compositions containing ketoconazole and medium-chain fatty acids and / or esters and other excipien...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com