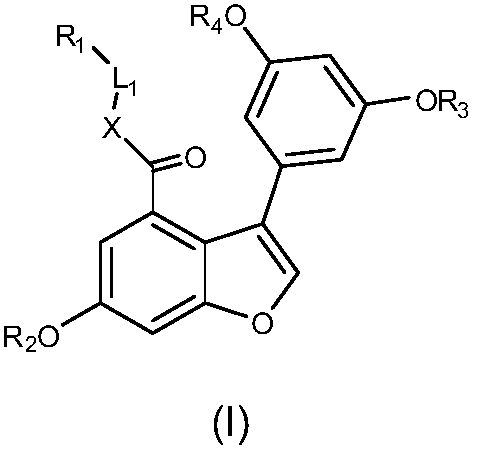
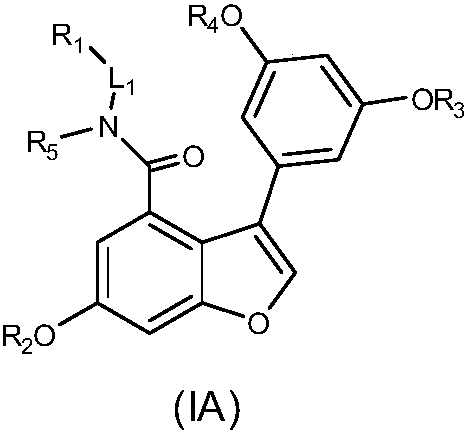
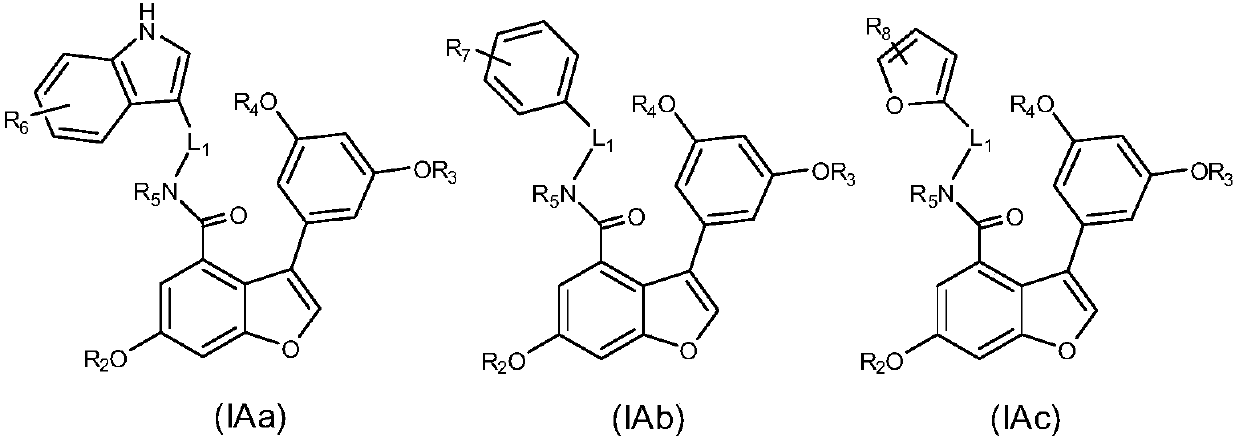
3-phenyl-7,8-dehydrogenated vitis amurensis vine pentosidine derivatives as well as preparation method and pharmaceutical composition thereof and application of derivatives and pharmaceutical composition
A technology of glupapentin and its derivatives, which is applied in the field of biomedicine and can solve problems such as structure-activity relationship optimization of compound systems that have not been reported in the literature.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0128] In order to further clarify the present invention, a series of examples are given below, these examples are completely illustrative, they are only used to specifically describe the present invention, and should not be construed as limiting the present invention.
[0129] The synthetic route of compound intermediate 1b in the embodiment:
[0130]
Embodiment 1
[0132] 3-(3,5-Dimethoxyphenyl)-6-methoxy-4-benzofurancarboxylic acid (1)
[0133] The synthetic route of compound 1:
[0134]
[0135] Compound 1b (10.0g, 29.2mmol) was added to 150mL THF, MeOH and H 2 In the mixed solution of O (1:1:1, v / v / v), stir to dissolve it, and add 1.17g of NaOH. The reaction mixture was heated to reflux for 12 h, and the reaction raw materials were hydrolyzed completely. Most of the solvent was distilled off the reaction solution under reduced pressure at 42°C, and 1 mol / L HCl solution was added dropwise until no white precipitate was precipitated. The reaction mixture was suction-filtered, washed with distilled water, and dried to obtain compound 1 (9.48 g, 98.9%) as a white powdery solid.
[0136] Compound 1: white powder. 1 H NMR (500MHz, acetone-d 6 )δ7.89(s,1H),7.37(d,J=2.0Hz,1H),7.31(d,J=2.0Hz,1H),6.54(d,J=2.0Hz,2H),6.43(t, J=2.0Hz,1H), 3.94(s,3H),3.78(s,6H); 13 C NMR (125MHz, acetone-d 6 ):δ168.07,161.41,158.52, 158.15,144.22,135.64,...
Embodiment 2
[0146] 3-(3,5-Dimethoxyphenyl)-6-methoxy-4-benzofurancarboxylic acid acetic anhydride (2)
[0147] Synthesized according to method B, the alcohol compound added was 4-acetamidophenoxyethyl ester (70.6 mg, 0.36 mmol), using petroleum ether: acetone (2:1) as a developing solvent, prepared and separated on a silica gel plate to obtain the target product, Yield 79.9%. The physicochemical parameters of compound 2 are as follows:
[0148] Compound 2: off-white solid, yield=39.1%. 1 H NMR (500MHz, acetone-d 6 ):δ7.89(s,1H),7.38(d,J=2.0Hz,1H),7.24(d,J=2.0Hz,1H),6.50(s,3H),3.93(s,3H),3.81 (s,6H),3.30(s,3H); 13 C NMR (125MHz, acetone-d 6 ):δ168.04,161.79(3×C), 158.61,157.97,144.10,135.80,127.09,123.82,118.81,113.72,107.15(2×C),100.17, 100.09,56.44,55.68(2×C),51. (+)-ESI m / z:393[M+Na] + ,409[M+K] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com