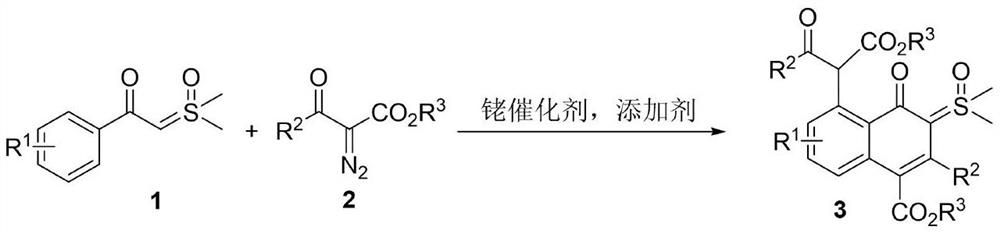
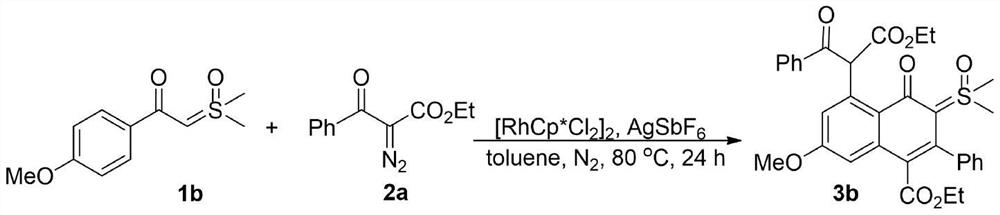
Synthesis of 4-oxo-5-(arylformyl acetate-2-yl)naphthalene-sulfoxide ylide hybrids
A technology of arylformyl acetate and arylformyl sulfoxide, which is applied in the field of synthesis of 4-oxo-5-naphthalene-sulfoxide ylide hybrid, and achieves simple operation process, high atom economy, and scope of application wide effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020]
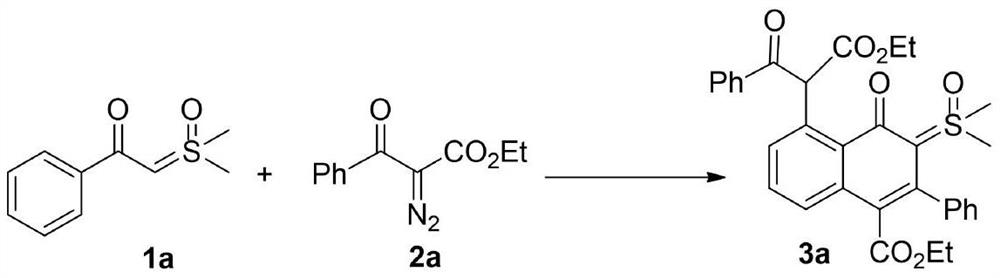
[0021] 1a (0.5mmol, 98mg), 2a (0.5mmol, 109mg), silver hexafluoroantimonate (27.5mg, 0.08mmol), [RhCp*Cl 2 ] 2 (12mg, 0.02mmol) and 1,2-dichloroethane (3mL), stirred at 80°C for 24h under nitrogen atmosphere. TLC detects that in addition to the main product, there is also a major impurity point generated. Then the reaction system was cooled to room temperature, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), the organic phases were combined, and washed with anhydrous Na 2 SO 4 After drying, the solvent was spin-dried and separated by silica gel column (petroleum ether / ethyl acetate=1 / 1) to obtain the product 3a (64 mg, 23%) as a pale yellow solid. The characterization data of this compound are as follows: 1 H NMR (600MHz, CDCl 3 )δ: 0.80(t, J=7.2Hz, 3H), 1.17(t, J=7.2Hz, 3H), 3.56(s, 3H), 3.58(s, 3H), 3.81(q, J=7.2Hz, 2H), 4.17(q, J=7.2Hz, 2H), 7.08(d, J=7.2Hz, 1H), 7.24-7.32(m, 7H), 7.36-7.42(m, 2H), 7.54(d,...
Embodiment 2
[0023] 1a (0.5mmol, 98mg), 2a (0.5mmol, 109mg), silver hexafluoroantimonate (27.5mg, 0.08mmol), [RhCp*Cl 2 ] 2 (12mg, 0.02mmol) and tetrahydrofuran (3mL), stirred at 80°C for 24h under nitrogen atmosphere. TLC detects that in addition to the main product, there is also a major impurity point generated. Then the reaction system was cooled to room temperature, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), the organic phases were combined, and washed with anhydrous Na 2 SO 4 After drying, the solvent was spin-dried and separated by silica gel column (petroleum ether / ethyl acetate=1 / 1) to obtain product 3a (39 mg, 14%) as a pale yellow solid.
Embodiment 3
[0025] 1a (0.5mmol, 98mg), 2a (0.5mmol, 109mg), silver hexafluoroantimonate (27.5mg, 0.08mmol), [RhCp*Cl 2 ] 2 (12mg, 0.02mmol) and acetonitrile (3mL), stirred at 80°C for 24h under nitrogen atmosphere. TLC detects that in addition to the main product, there is also a major impurity point generated. Then the reaction system was cooled to room temperature, 10 mL of saturated brine was added to quench the reaction, extracted with ethyl acetate (10 mL×3), the organic phases were combined, and washed with anhydrous Na 2 SO 4 After drying, the solvent was spin-dried and separated by silica gel column (petroleum ether / ethyl acetate=1 / 1) to obtain product 3a (28 mg, 10%) as a pale yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com