Application of MFAP5 in preparing preparation for screening markers of people sensitive to alpha interferon intervention in liver cancer
A technology of alpha interferon and markers, applied in the field of biomedical clinical detection, can solve the problems that the role of MFAP5 needs to be elucidated, the overall curative effect of liver cancer patients cannot fully meet the clinical requirements, and there is no interferon alpha intervention in sensitive groups of liver cancer. The effect of high accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 kit composition:
[0024] Liver cancer alpha interferon treatment sensitive population screening diagnostic kit (10 servings)
[0025] [(1) 2.5ug / ml human soluble MFAP5 antibody 0.5ml
[0026] (2) HRP-labeled goat anti-mouse IgG 0.5ml
[0027] (3) DAB Chromogenic Solution (Wuhan Boster Company) 0.5ml
[0028] (4) Hematoxylin counterstain solution 0.5ml
[0029] (5) 3%H 2 o 2 0.5ml
[0030] How to use the kit:
[0031] (1) Baking sheet: 91.7°C, 30 minutes; take it out and place it in xylene I;
[0032] (2) Dewaxing: successively insert xylene I and II for 10 minutes each;
[0033] (3) Alcohol hydration: put different concentrations of alcohol (100%, 95%, 80%, 75%) in sequence for 5 minutes each;
[0034] (4) Wash the slices with PBS, 5min, 3 times. Inactivation of endogenous peroxidase: Each tablet was given 200ul H2O2 (6%) and placed in a wet box for 15 minutes. Wash the slides with PBS, 5min, 3 times;
[0035] (5) Heat repair: Insert slices in...
Embodiment 2
[0040] Kit for the detection of liver cancer clinical samples
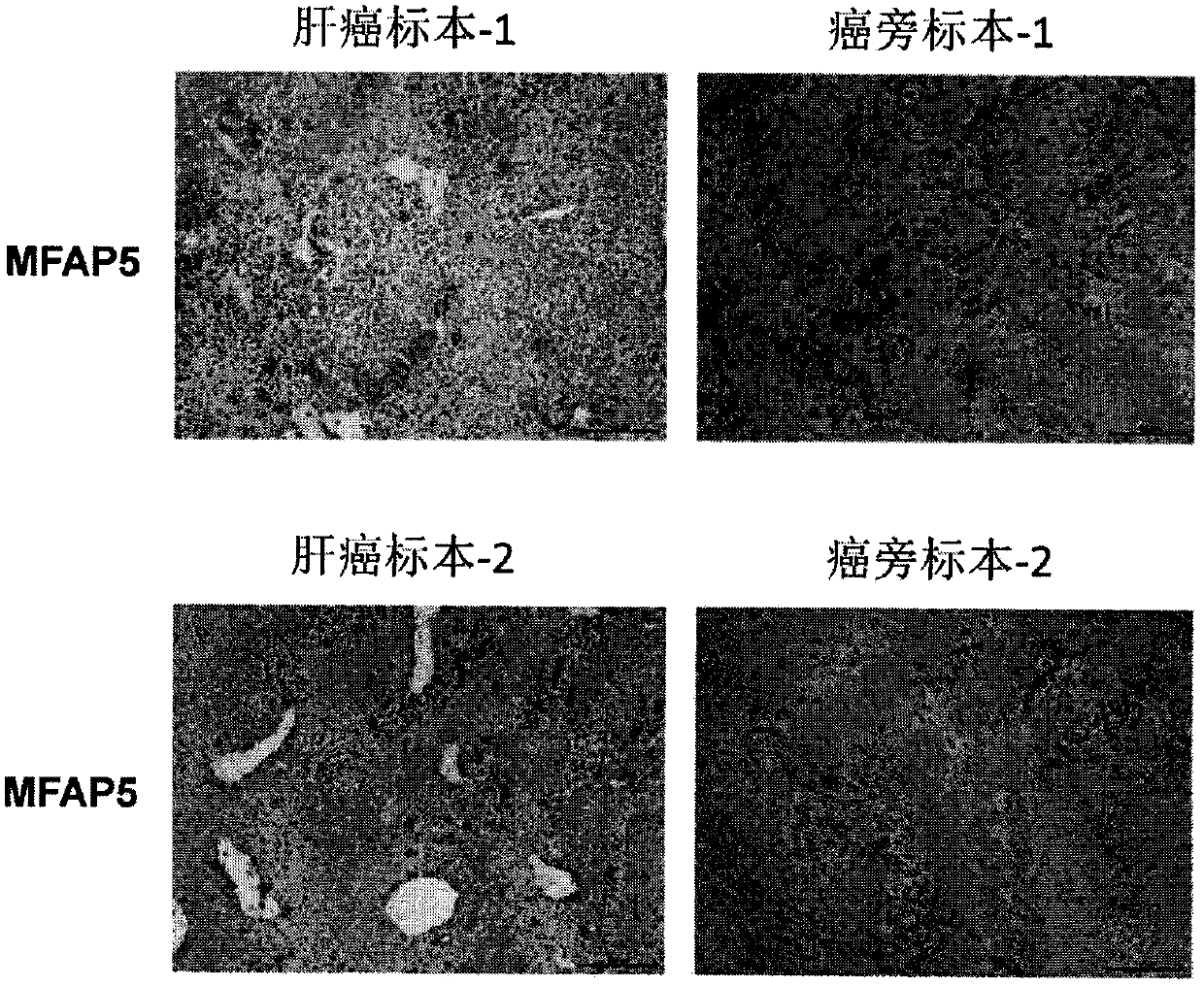
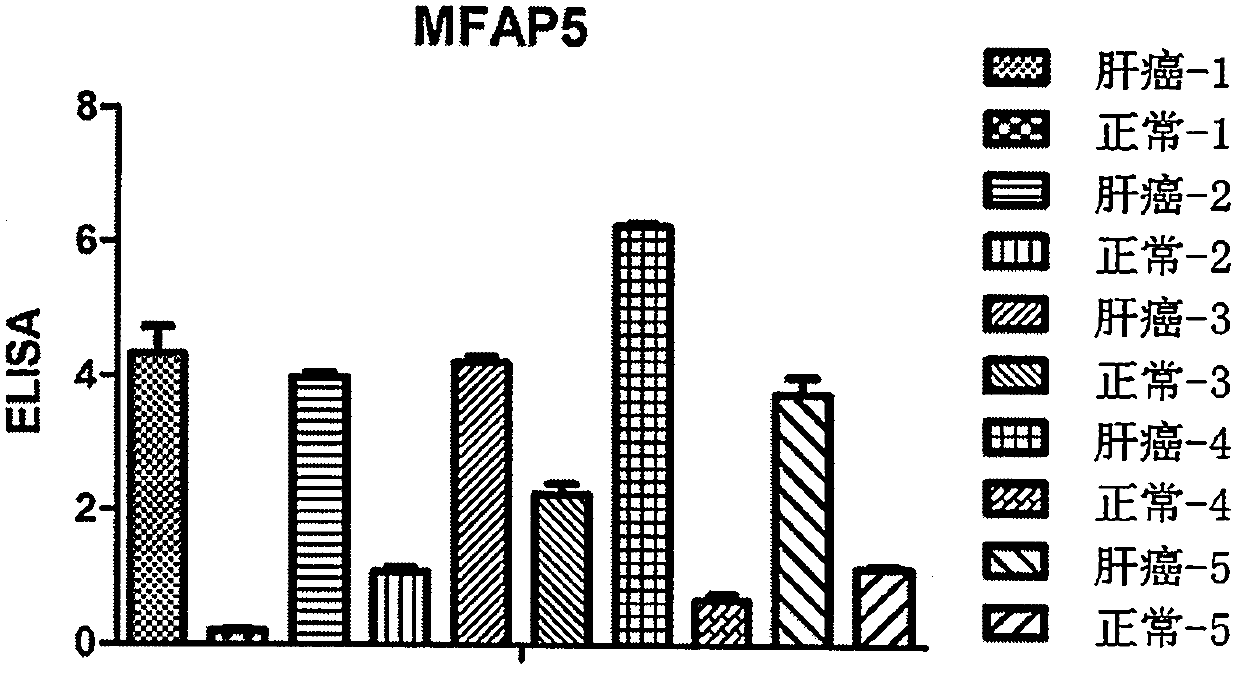
[0041] 170 clinical specimens of liver cancer were collected, including liver cancer tissues and adjacent tissues (such as figure 1shown), experiment was carried out according to the instructions of the kit, and the results showed that according to the degree of staining + (<20%), ++ (20-50%), +++ (51-75%), ++++ ( 76-100%) grade 4, and finally analyzed with statistical software, the positive rate of MFAP5 in liver cancer reached 41.81%, with a significant difference.
Embodiment 3
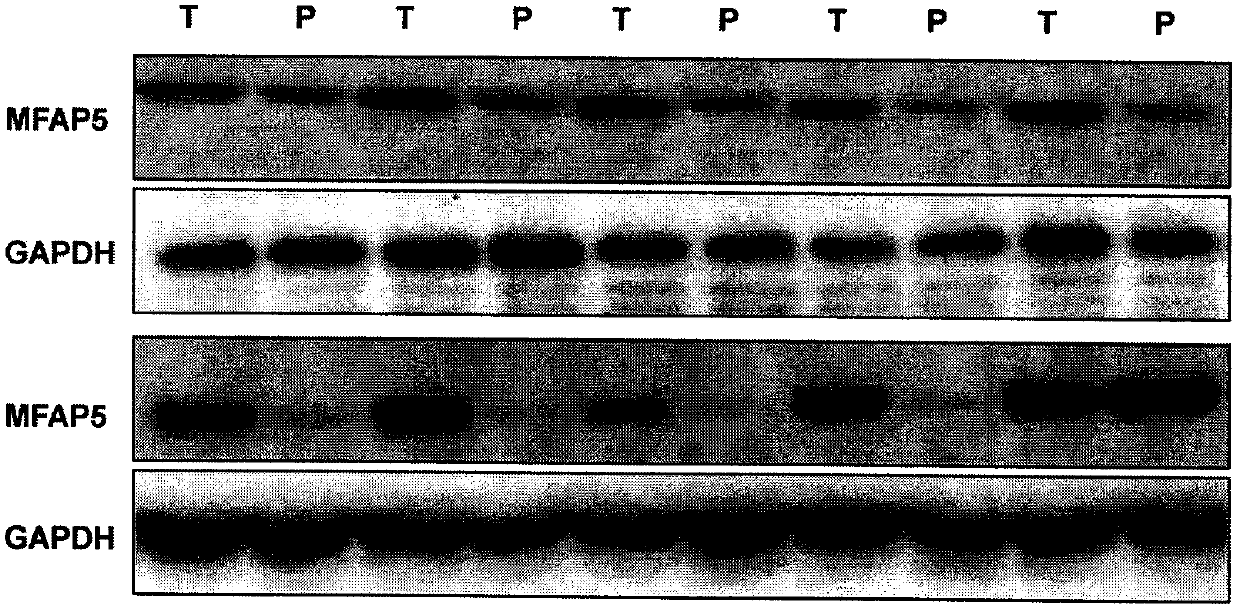
[0043] Detection of Liver Cancer Clinical Samples by Western blot
[0044] 10 pair of liver cancer operation samples collected, Western blot is tested, and the result shows that the MFAP5 positive specimen identified in embodiment 2 meets Western blot detection result (such as figure 2 shown).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com