Anticancer pharmaceutical application of glufosfamide
A technology of glufosfamide and cancer, applied in the field of glufosfamide, can solve the problems such as no development and listing, no significant increase in overall survival rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
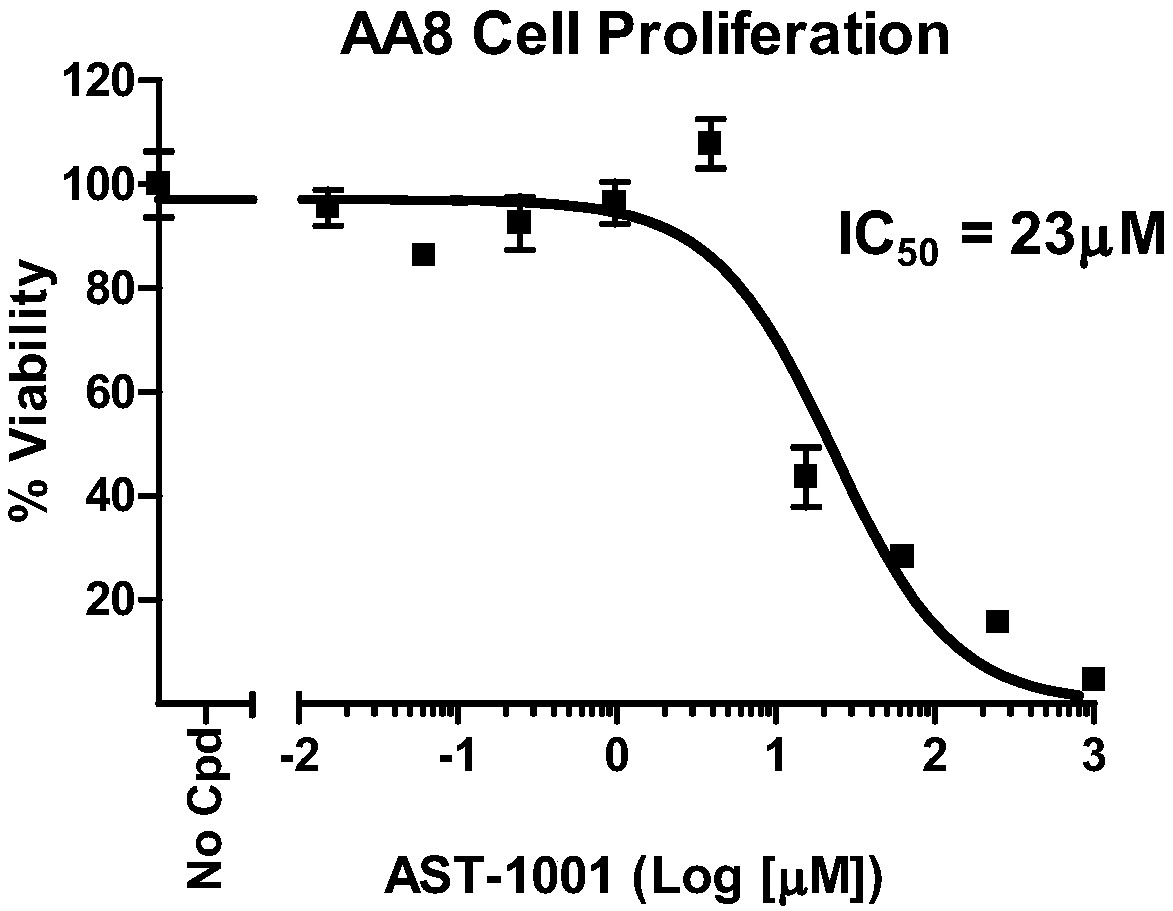
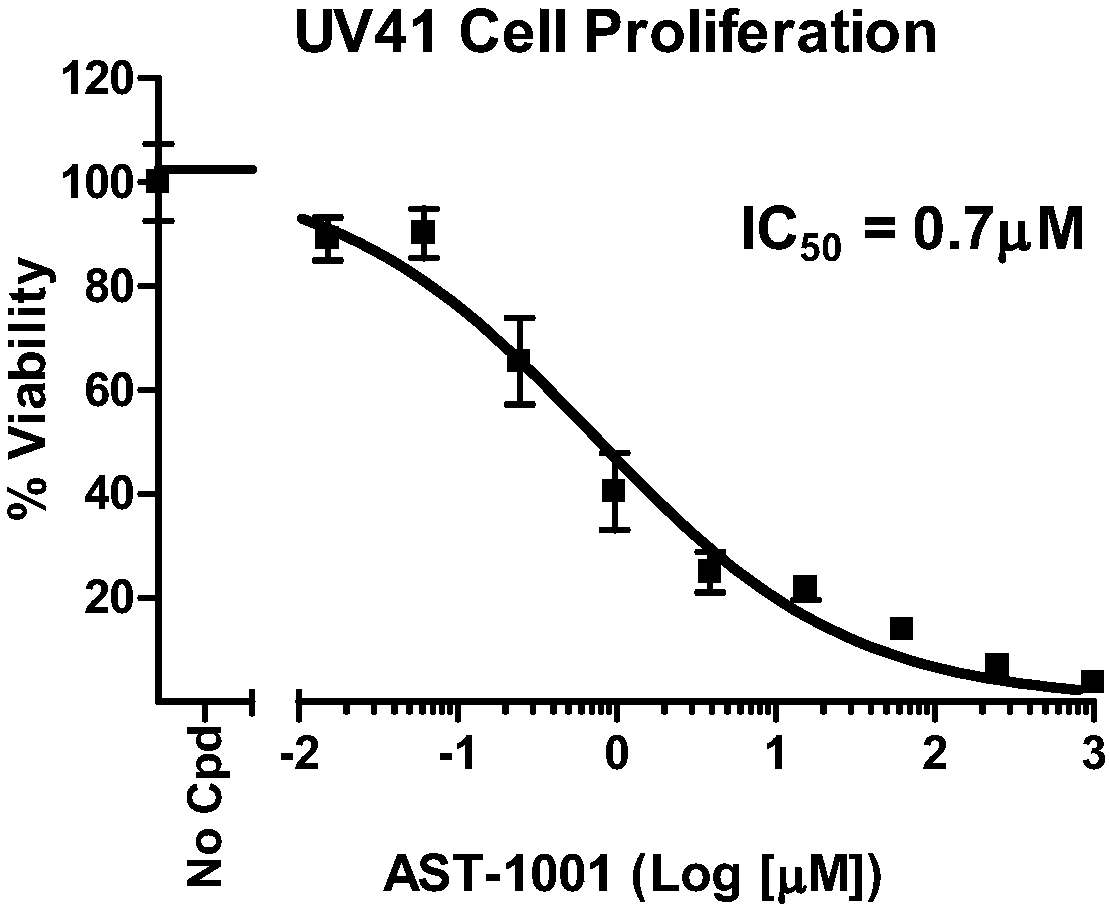
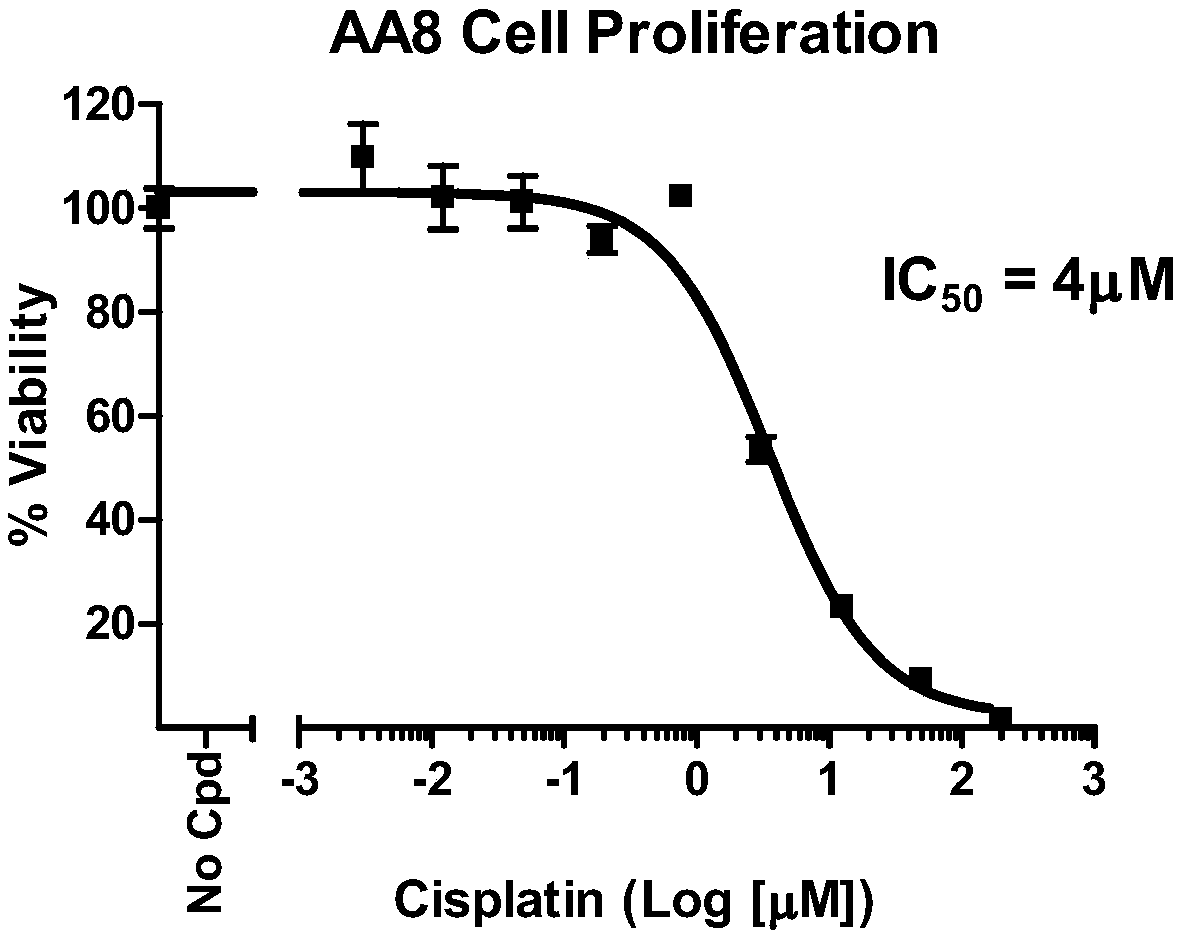
[0180] In vitro cell experiment
[0181] 1. Materials and methods
[0182] 1.1. Materials and instruments
[0183] AA8 cell line, purchased from American type culture collection (ATCC#CRL-1859);
[0184] UV41 cell line, purchased from American type culture collection (ATCC#CRL-1860);
[0185] MEM-alphamedium medium, purchased from Fisher Reagent Company (Fisher#12-561-056);
[0186] Fetal bovine serum (FBS for short), purchased from ThermoFisher (ThermoFisher#26140-079);
[0187] Penicillin streptomycin solution-double antibody Penn-step, purchased from Hyclone Company (Hyclone#SV30010);
[0188] AlamarBlue reagent was purchased from ThermoFisher (ThermoFisher #DAL1100).
[0189] 1.2. Test compound
[0190] compound
Dissolving solvent
Test concentration (μM / L)
dilute solution
Glufosfamide
DMSO
0.015-1000
Media solution containing 0.5% DMSO
DMSO
0.003-200
Medium solution containing 0.5% DMSO
[0191]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com