A blood cell lysate and method for extracting nucleic acid in blood using the lysate
A blood cell and lysate technology, applied in biochemical equipment and methods, microbial measurement/inspection, DNA preparation, etc., can solve problems such as low efficiency, affecting follow-up tests, and easy contamination, achieving high yield and saving detection time , the effect of not easy to pollute
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
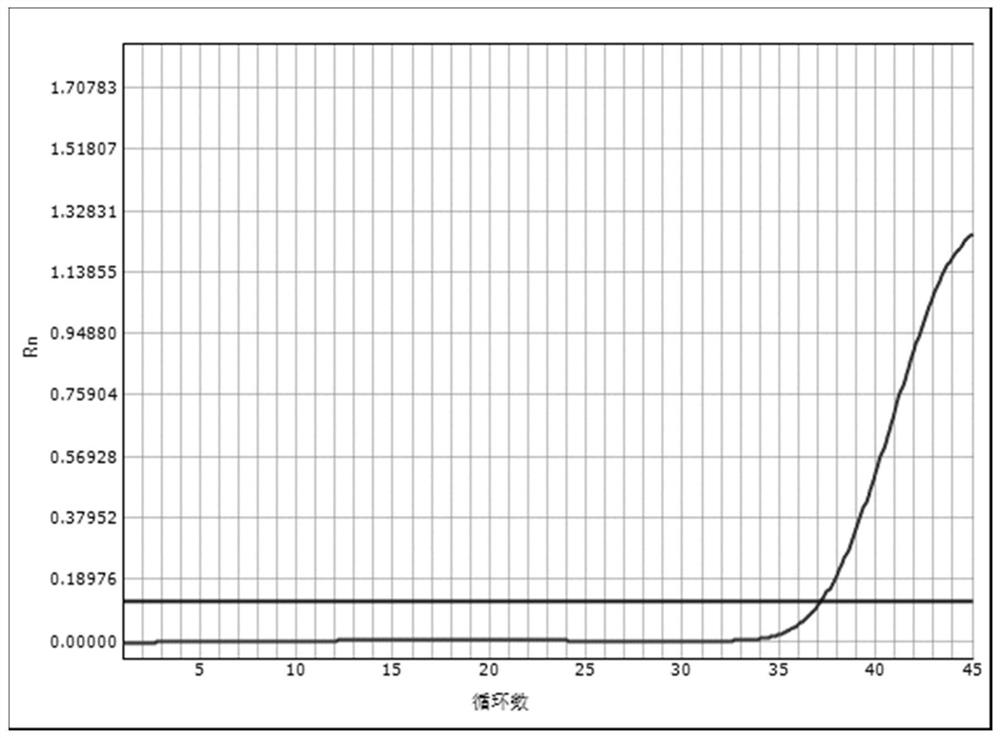
Embodiment 1
[0058] Embodiment 1: Real-time fluorescent quantitative PCR detection of EBV DNA
[0059] The reagents prepared according to the above-mentioned lysate solution mixed with heparin magnetic beads scheme 1) were used to confirm the clinical diagnosis and the quantitative value of EBV DNA after detection was 2×10 2 IU / ml patient blood is operated as follows:
[0060] Step 1) Put the magnetic bead chromatography column in a nuclease-free 1.5mL centrifuge tube,
[0061] Step 2) Add 20 μl of the lysate mixed with heparin magnetic beads to the bottom of the magnetic bead chromatography column tube; take 120 μl of the sample to be tested and add it to the lysate mixed with heparin magnetic beads, mix well, and stand at 95°C for 10 minute;
[0062] Step 3) Move the sleeve to a centrifuge for 5 minutes at 15,000 rpm, and the precipitated liquid is the extraction of nucleic acid.
[0063] Step 4) Taq DNA polymerase of 4.0 μl (0.5U / μl) unit is mixed with 36.0 μl PCR reaction solution (...
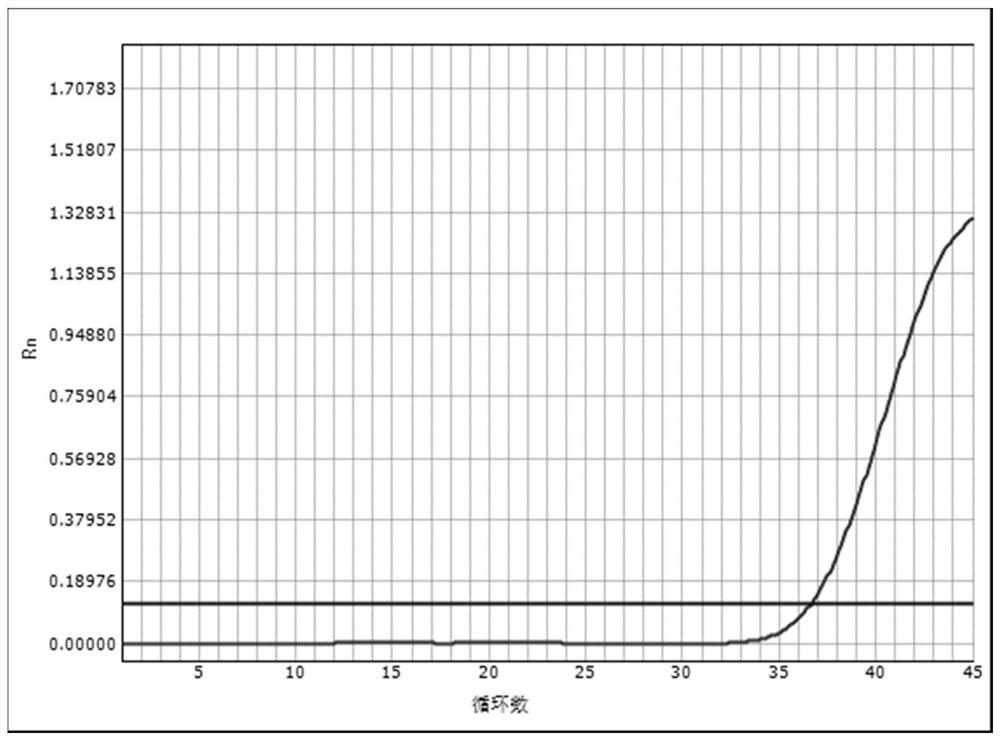
Embodiment 2
[0068] Embodiment 2: Real-time fluorescent quantitative PCR detection of EBV DNA
[0069] The reagents prepared by scheme 2) respectively have a clear clinical diagnosis and a quantitative value of 2×10 EBV DNA after testing. 2 IU / ml hepatitis B patient's serum carries out the operation of following steps:
[0070] Step 1) Put the magnetic bead chromatography column in a nuclease-free 1.5mL centrifuge tube,
[0071] Step 2) Add 20 μl of the lysate mixed with heparin magnetic beads to the bottom of the magnetic bead chromatography column tube; take 120 μl of the sample to be tested and add it to the lysate mixed with heparin magnetic beads, mix well, and stand at 85°C for 5 minute;
[0072] Step 3) Move the cannula to a centrifuge for 10 minutes at 10,000 rpm, and the precipitated liquid is the extraction of nucleic acid.
[0073] Step 4) Taq DNA polymerase of 4.0 μl (0.5U / μl) unit is mixed with 36.0 μl PCR reaction solution (the reaction solution is to include concentration...
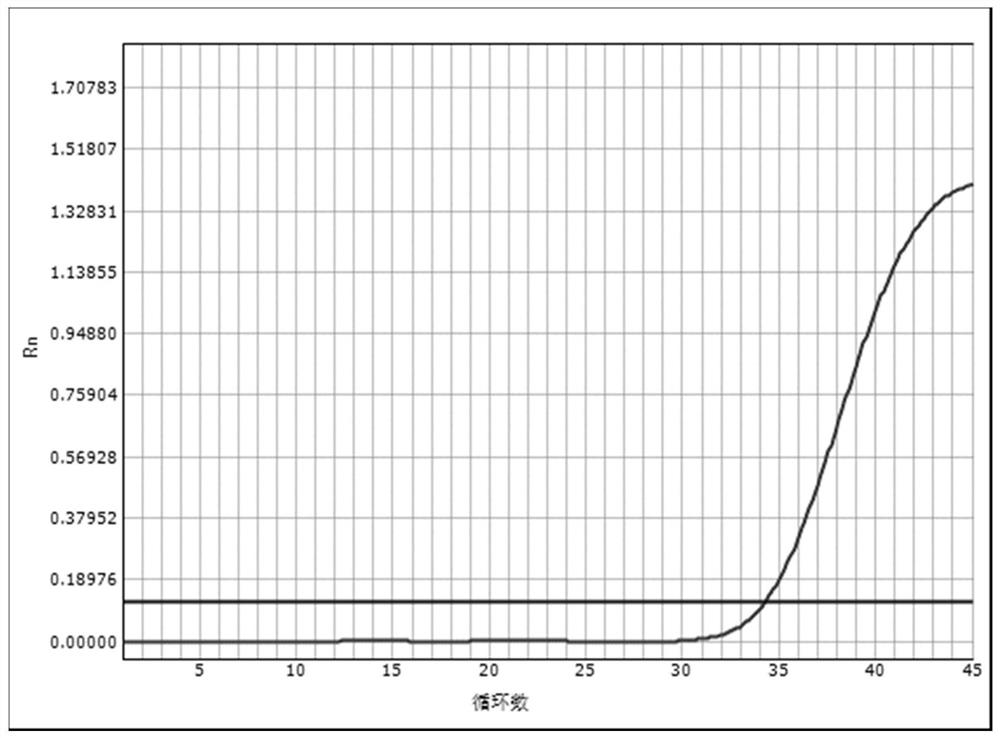
Embodiment 3
[0078] Embodiment 3: Real-time fluorescent quantitative PCR detection of EBV DNA
[0079] The reagents prepared according to the above-mentioned lysate solution mixed with heparin magnetic beads scheme 1) were used to confirm the clinical diagnosis and the quantitative value of EBV DNA after detection was 2×10 2 IU / ml patient blood is operated as follows:
[0080] Step 1) Put the magnetic bead chromatography column in a nuclease-free 1.5mL centrifuge tube,
[0081] Step 2) Add 20 μl of the lysate mixed with heparin magnetic beads to the bottom of the magnetic bead chromatography column; take 120 μl of the sample to be tested and add it to the lysate mixed with heparin magnetic beads, mix well, and stand at 90°C 8 minutes;
[0082] Step 3) Move the cannula to a centrifuge for 5 minutes at 12000 rpm, and the precipitated liquid is the extraction of nucleic acid.
[0083] Step 4) Taq DNA polymerase of 4.0 μl (0.5U / μl) unit is mixed with 36.0 μl PCR reaction solution (the react...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com