Rapid prototyping and in vitro modeling of patient-specific coronary artery bypass grafts
A patient-customized, coronary-based technology with applications in character and pattern recognition, prosthetics, and recognition of medical/anatomical patterns
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
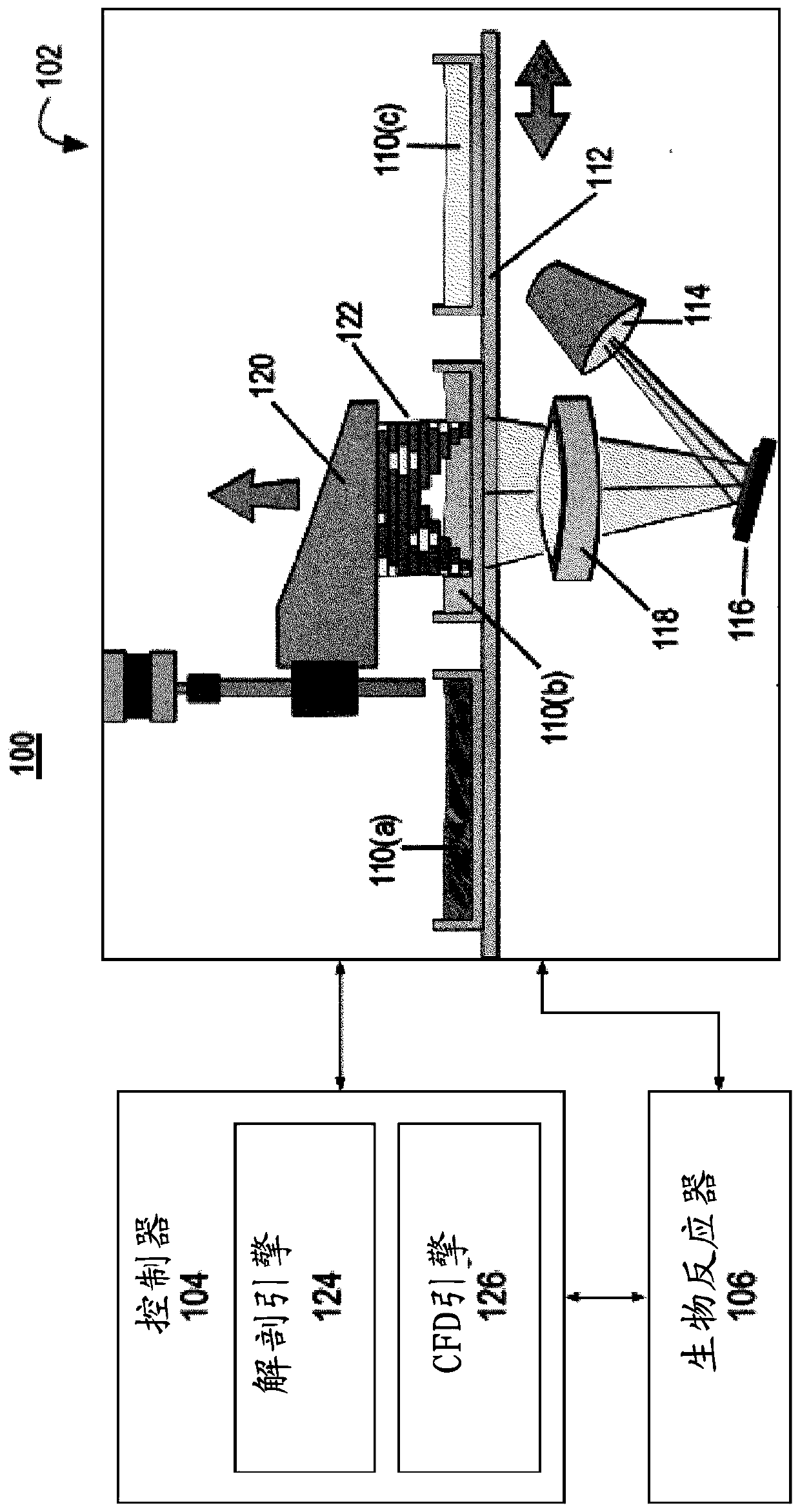
[0051] Example 1: CTA of the coronary arteries can be a non-invasive alternative to invasive coronary angiography (ICA). In some implementations, dissection engine 124 is configured to receive motion-free images (eg, CTA images) at an isotropic spatial resolution of about 500 μm. In one example, system 100 performed CTA prior to ICA on 230 patients without regard to body mass index or heart rate. For stenosis severity, CTA showed 94%, 83%, 48% and 99% sensitivity, specificity, positive predictive value and negative predictive value compared with ICA, respectively.
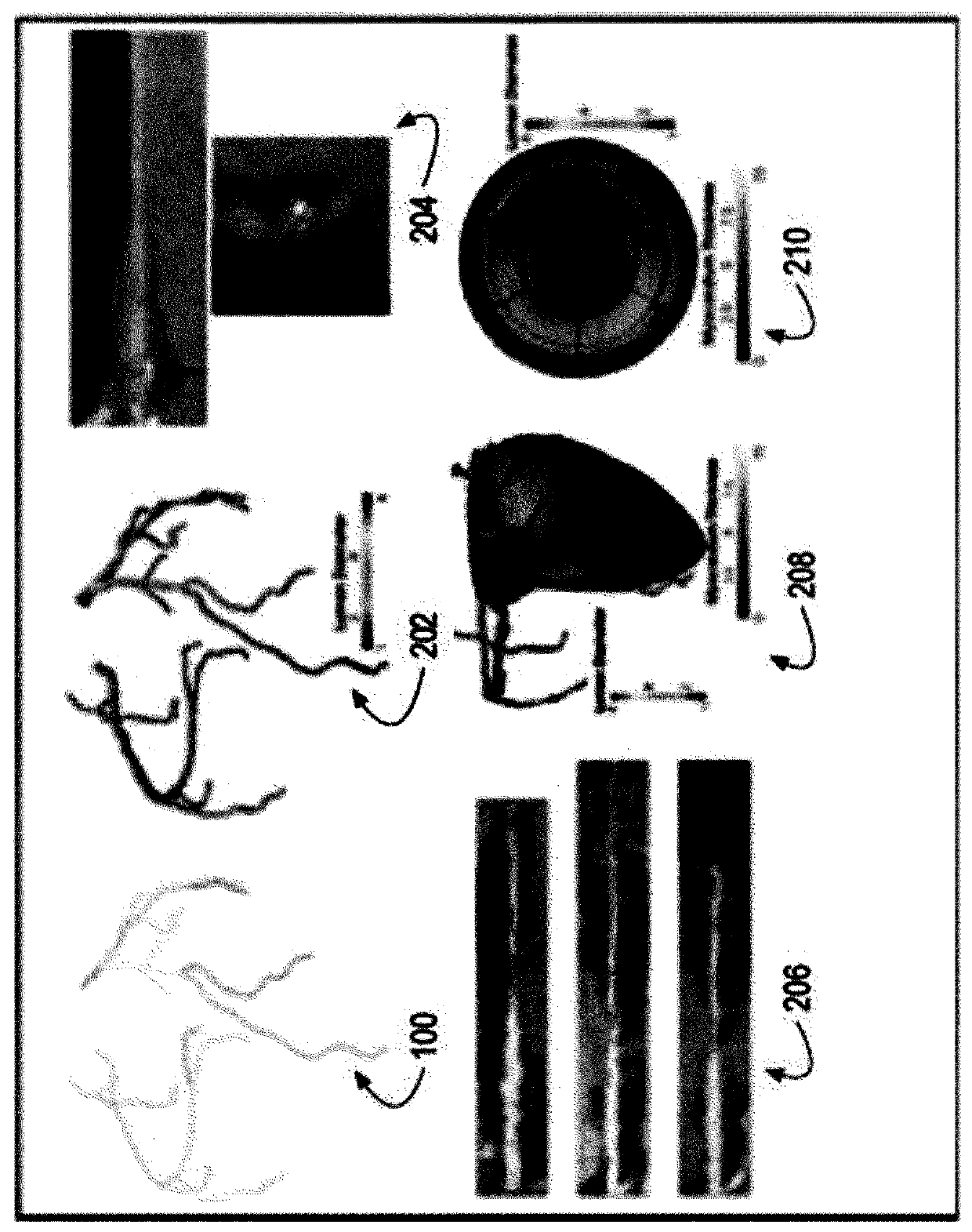
[0052] Anatomy engine 124 may receive CTA data to generate 3D coronary artery geometry for image-based modeling of coronary artery anatomy. The dissection engine 124 determines an arterial centerline extraction 200 . Using centerline extraction, lumen segmentation and stenosis can be performed 202 . The dissection engine 124 may also perform vessel wall segmentation and plaque detection 204 and arterial co-regis...
example 2
[0053] Example 2: In some implementations, the anatomy engine 124 can generate anatomical information about a patient-customized SDVG. CFD engine 126 may generate hemodynamic curves by performing computational fluid dynamics on anatomical information. Typically, hemodynamic profiles can include clinical factors, design factors, or other factors such as flow oscillations, flow stagnation, flow energy loss, wall mechanics, shear levels, and graft-arterial compliance mismatch. The CFD engine 126 can compute hemodynamic (eg, physiological) data of aortic and coronary blood flow and pressure. image 3 The pressure, velocity and flow calculated by the CFD engine 126 in the patient-customized SDVG 122 are shown. In some embodiments, the CFD engine 126 can plan a "virtual" revascularization strategy by selecting coronary vessels and locations within vessels to revascularize flow characteristics and ischemia reduction. Figure 4 A patient-customized SDVG 122 after percutaneous corona...
Embodiment 4
[0060] Example 4: In vitro measurements in a benchtop flow circulator using a patient-customized SDVG physical model fabricated by the printing subsystem 102 (eg, a multi-material high-resolution 3D printing system). Experiments demonstrate that the system 100 can generate complex 3D arterial geometries with continuously spatially varying mechanical properties. Figure 5A A custom SDVG 122 is shown as a 3D printed physical model for a patient. 3D printed physical models allow the evaluation of patient-specific, graft-specific and surgical technique-related factors affecting SDVG hemodynamics and wall mechanics by 3 different methods: (i) intravascular pressure and flow sensors, (ii) particle Image velocimetry (PIV) and (iii) embedded soft strain sensors.
[0061] Figure 5B and 6 Shown is an extracorporeal flow circulatory system constructed to approximate coronary blood flow physiology to determine input defined flow velocity (Q), proximal (Pa) and distal (Pd) pressure and...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com