Oxygen nitrogen heterocyclic heptane derivative containing exocyclic double bond and preparation method thereof
A technology for oxazepane and derivatives is applied in the field of oxazepane derivatives and their preparation, and can solve the problems of low substrate universality, high transition metal prices, harsh reaction conditions and the like, To achieve the effects of complex and novel structure, short reaction time and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
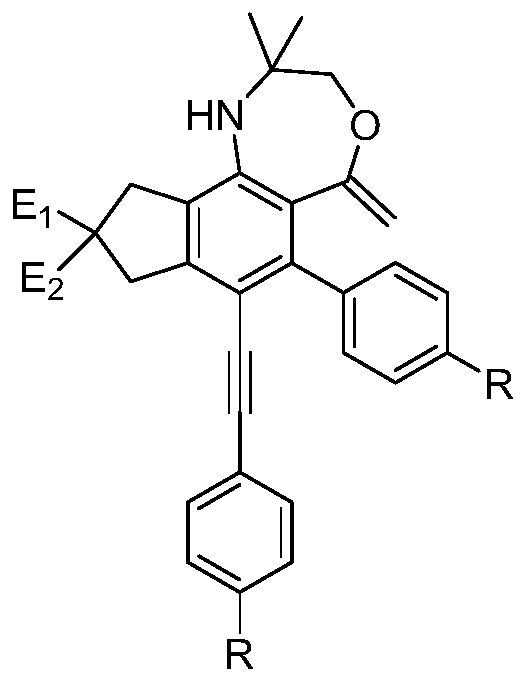
[0044] A kind of oxazepane derivative containing exocyclic double bond, its structural formula is:
[0045]
[0046] The preparation method of the above-mentioned oxazepane derivatives containing exocyclic double bonds comprises the following steps:
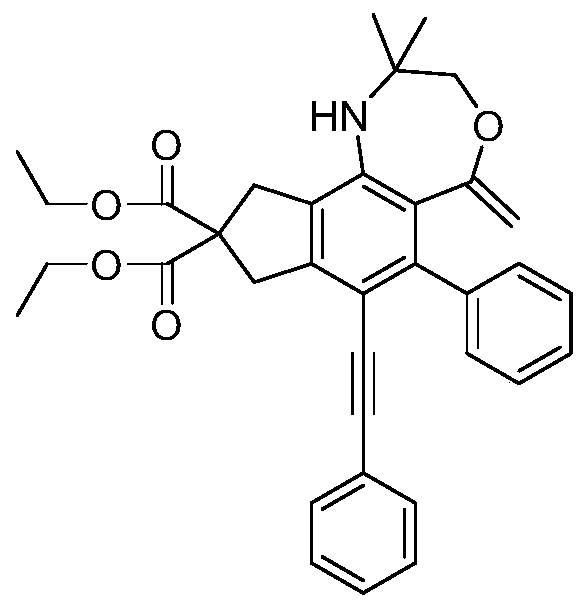
[0047] (1) With 830mmol sodium hydride as a catalyst, 200mmol diethyl malonate and 440mmol propargyl bromide were added to 210mL anhydrous acetonitrile in an ice-water bath, stirred and reacted for 8 hours, the product was washed with water, extracted with ethyl acetate, and Press spin dry, obtain product, i.e. compound 1, structural formula
[0048] (2) Mix 80mmol of compound 1 prepared in step (1) with 200mmol of phenylethynyl bromide in Pd(PPh 3 ) 2 Cl 2 / CuI anhydrous and oxygen-free catalytic system (2.56mmol / 0.85mmol), the molar ratio of Pd(PPh 3 ) 2 Cl 2 : CuI=3:1, with 336mmol triethylamine as base, 150mL anhydrous acetonitrile as solvent, stirring and reacting at room temperature for 12 hours, the product was ...
Embodiment 2
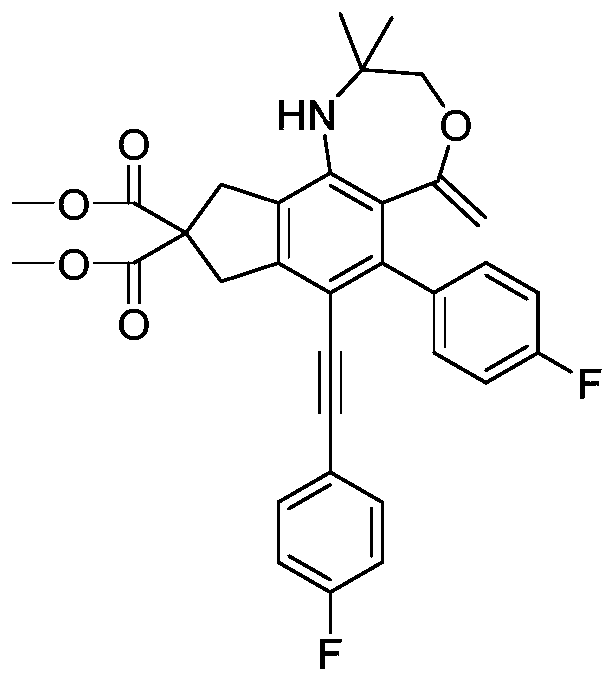
[0055] A kind of oxazepane derivative containing exocyclic double bond, its structural formula is:
[0056]
[0057] The preparation method of the above-mentioned oxazepane derivatives containing exocyclic double bonds comprises the following steps:
[0058] (1) With 830mmol sodium hydride as a catalyst, 200mmol dimethyl malonate and 440mmol propargyl bromide were added to 210mL anhydrous acetonitrile in an ice-water bath, stirred and reacted for 8 hours, the product was washed with water, extracted with ethyl acetate, and Press spin dry, obtain product, i.e. compound 1, structural formula
[0059] (2) Mix 80mmol of compound 1 prepared in step (1) with 200mmol p-fluorophenylethynyl bromide in Pd(PPh 3 ) 2 Cl 2 / CuI in anhydrous and oxygen-free catalytic system (2.56mmol / 0.85mmol), molar ratio
[0060] Pd(PPh 3 ) 2 Cl 2 : CuI=3:1, with 336mmol triethylamine as base, 150mL anhydrous acetonitrile as solvent, stirring and reacting at room temperature for 12 hours, the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com