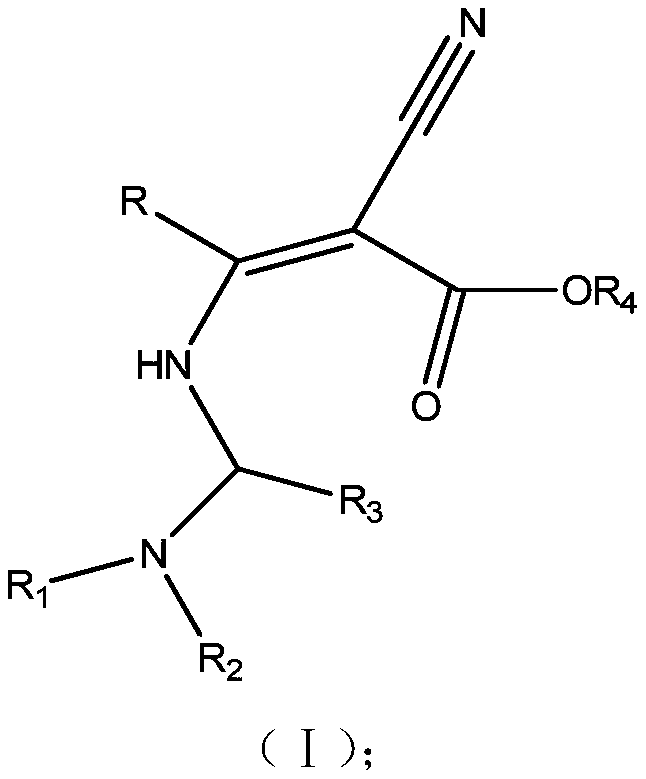
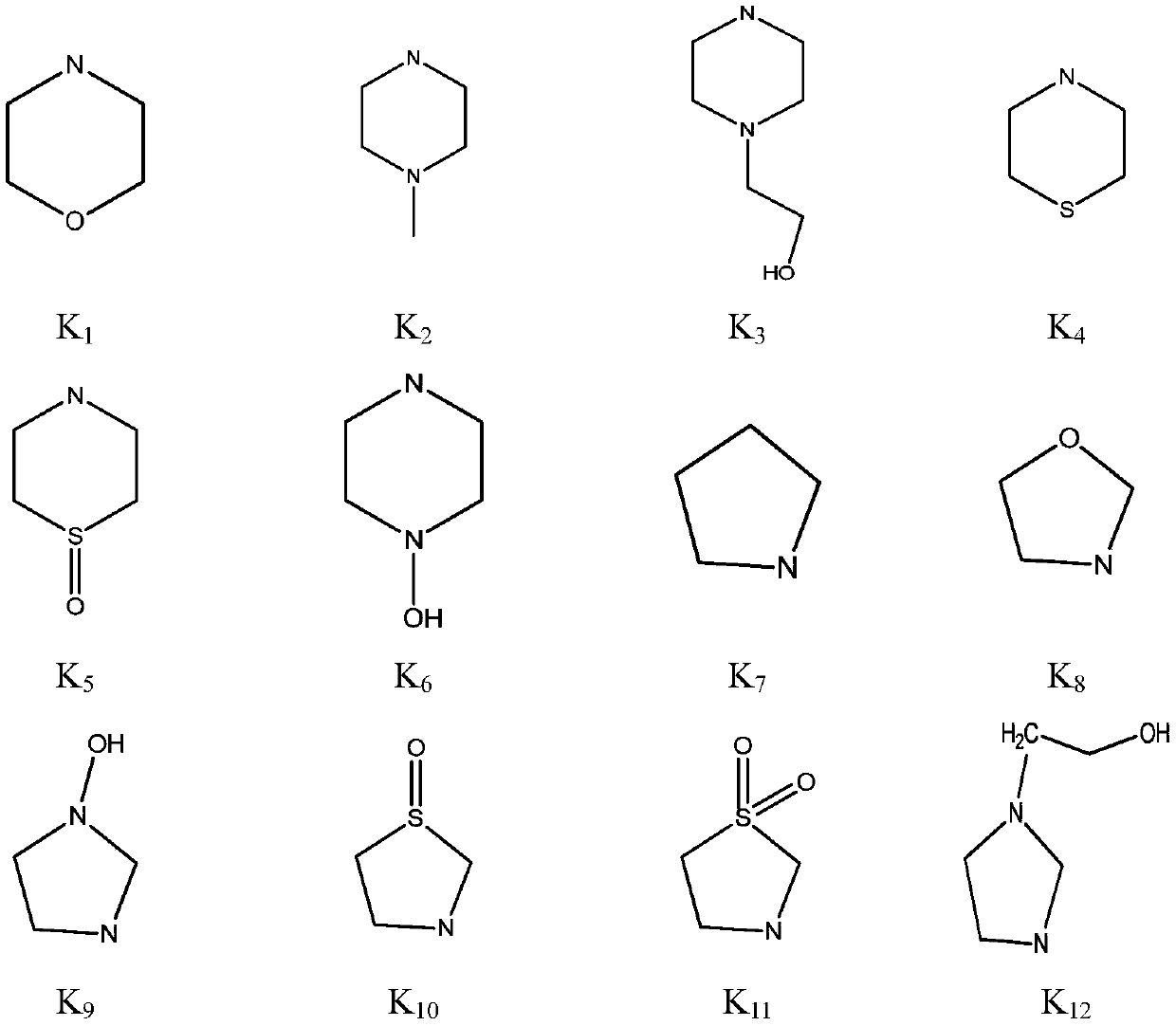
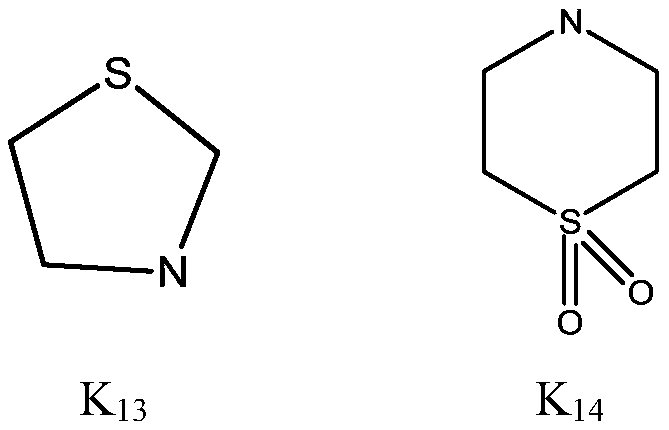
3-amino-2-cyanoacrylate type compound and application thereof
A technology of cyanoacrylates and compounds, applied in the field of 3-amino-2-cyanoacrylate compounds, which can solve the problems of limited application range and low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078]
[0079] In a four-neck flask equipped with a thermometer, mechanical stirring, reflux condenser, and dropping funnel, suspend 21.2 g (0.1 mol) of cyclostrobin (2-cyano-3-amino-3-phenylacrylate ethyl ester) In 600g of ethylene dichloride solution, 17.4g (0.2mol) morpholine joins in this solution, and system is warmed up to 70 ℃, and at this temperature, the formaldehyde (0.2mol) aqueous solution of 17.5g35% in 30 minutes is added dropwise to In the reaction solution, after the dropwise addition is completed, the reaction is continued at this temperature for 1 hour, the temperature is lowered to 0 degrees, the unreacted substances are filtered off, the phases are separated, and the dichloroethane phase is concentrated to obtain the Mannich base of cyclostrobin. Recrystallization obtained 10 g of morpholine Mannich base of cyclostrobin, ie compound NO.1.
Embodiment 2
[0081]
[0082] In a four-necked flask equipped with a thermometer, mechanical stirring, reflux condenser and dropping funnel, 2.12g (0.01mol) cyclostrobin (2-cyano-3-amino-3-phenylacrylate ethyl ester) Suspended in 60g of toluene solution, 1.42g (0.02mol) tetrahydropyrrole was added to the solution, the system was heated up to 110°C, at this temperature, 1.92g of furfural (0.2mol) was added dropwise to the reaction solution within 30 minutes After the dropwise addition, the reaction was continued at this temperature for 1 hour, the temperature was lowered to 0°C, the unreacted substances were filtered off, the phases were separated, and the toluene phase was concentrated and separated by column chromatography to obtain 0.5 g of compound NO.8.
Embodiment 3
[0084]
[0085] In a four-neck flask equipped with a thermometer, mechanical stirring, reflux condenser, and dropping funnel, suspend 2.12 g (0.01 mol) of cyclostrobin (2-cyano-3-amino-3-phenylacrylate ethyl ester) In 60g of toluene solution, 1.74g (0.02mol) of morpholine was added to the solution, and the system was heated to 110°C. At this temperature, 2.14g (0.2mol) of pyridine-4 formaldehyde was added dropwise to the reaction within 30 minutes. solution, after the dropwise addition was completed, the reaction was continued at this temperature for 4 hours, the temperature was lowered to 0°C, the unreacted substances were filtered off, the phases were separated, and the toluene phase was concentrated and then separated by column chromatography to obtain 0.6g of Mannystrobin of cyclostrobin. Xi base, compound NO.15.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com