Unsaturated carbonyl compounds as well as preparation method and application thereof
A carbonyl compound and unsaturated technology, which is applied in the field of unsaturated carbonyl compounds and their preparation, can solve the problems of harsh synthesis conditions of unsaturated carbonyl compounds, synthetic methods not universally applicable, complex processes, etc., and achieve a safe and controllable reaction process , avoid strong oxidative conditions, high application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] In one embodiment, the preparation method of the unsaturated carbonyl compound includes the following steps:
[0078] S01: Provide γ,δ-unsaturated carbonyl compound A represented by the following structural formula:
[0079]
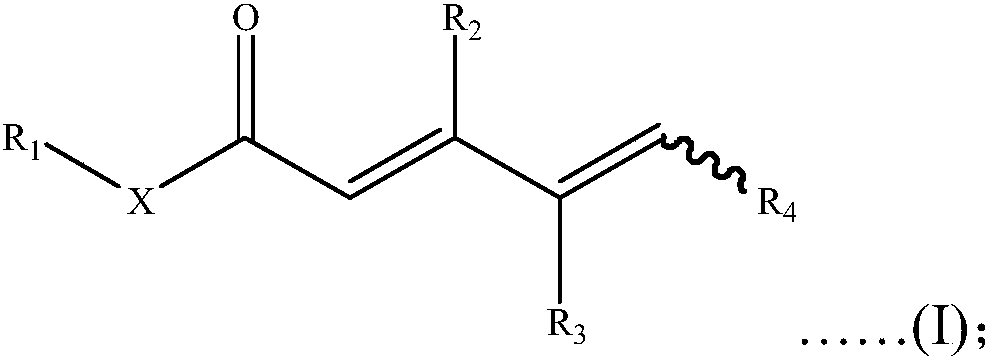
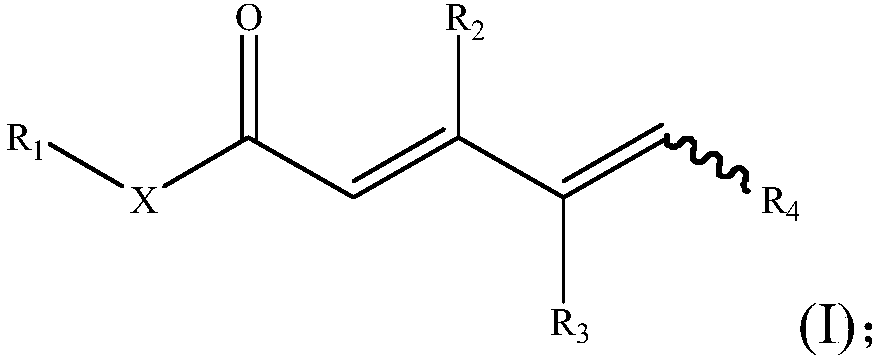
[0080] S02: The γ, δ-unsaturated carbonyl compound A is added to a reaction system containing transition metal catalysts, additives and oxidants, and reacted at 10 to 150°C to obtain the following general structural formula as shown in formula (I) Of unsaturated carbonyl compounds,
[0081]
[0082] Specifically, in the above step S01, the R in the γ, δ-unsaturated carbonyl compound A 1 , R 2 , R 3 And R 4 Are the same or different hydrogen atoms, C 1 -C 20 Alkyl, C 1 -C 20 Heteroalkyl, C 3 -C 20 Cycloalkyl, C 3 -C 20 Heterocycloalkyl, C 2 -C 20 Alkenyl, C 2 -C 20 Heteroalkenyl, C 3 -C 20 Cycloalkenyl, C 3 -C 20 Heterocyclenyl, C 2 -C 20 Alkynyl, C 2 -C 20 Heteroalkynyl, C 3 -C 20 Cycloalkynyl, C 3 -C 20 Heterocycloalkynyl, C 1 -C 20 Alkoxy, aryl, substi...
Embodiment 1
[0113] This Example 1 provides a (E)-N-(4-methoxybenzyl)-2,4-dienepentanamide and a preparation method thereof.
[0114] The structural formula of (E)-N-(4-methoxybenzyl)-2,4-dienpentylamide is shown in the following molecular structure I1:
[0115]
[0116] The preparation steps are as follows:
[0117] In a dry 10mL test tube, add transition metal iridium catalyst (0.002mmol, 0.02eq), silver tetrafluoroborate additive (0.005mmol, 0.05eq), copper acetate monohydrate (0.004mmol, 0.04eq), corresponding N-(4 -Methoxybenzyl)-4-enepentanamide substrate and 1.0 mL of anhydrous 1,4-dioxane. The reaction tube was sealed and the air was replaced six times. The resulting mixture was stirred at 60°C for 12 hours.
[0118] After the reaction, the reaction solution was filtered through a glass dropper containing silica gel, washed with dichloromethane, and the filtrate was spin-dried to obtain the target product I1, a white solid, with a yield of> 99%.
[0119] NMR characterization analysis, the r...
Embodiment 2
[0121] This example 2 provides a (2E, 4E)-N-(thiophen-3-methylene)-2,4-diene octamide and a preparation method thereof.
[0122] The structural formula of the (2E,4E)-N-(thiophen-3-methylene)-2,4-dienoctamide is shown in the following molecular structural formula I2:
[0123]
[0124] The preparation steps are as follows: In a dry 10mL test tube, add transition metal iridium catalyst (0.002mmol, 0.02eq), silver tetrafluoroborate additive (0.005mmol, 0.05eq), copper acetate monohydrate (0.004mmol, 0.04eq), corresponding (E)-N-(thiophen-3-methylene)-4-enoctamide substrate and 1.0 mL of anhydrous 1,4-dioxane, the reaction tube is sealed and the air is replaced six times, the resulting mixture Stir at 60°C for 20 hours.
[0125] After the reaction, the reaction solution was filtered through a glass dropper containing silica gel, washed with dichloromethane, and the filtrate was spin-dried to obtain the target product I2, a white solid, with a yield of 95%.
[0126] NMR characterization a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com