Unsaturated carbonyl compound and its preparation method and application
A carbonyl compound and unsaturated technology, which is applied in the field of unsaturated carbonyl compounds and their preparation, can solve the problems of harsh synthesis conditions of unsaturated carbonyl compounds, unapplicable synthesis methods, complicated processes, etc., and achieves a safe and controllable reaction process. , Avoid strong oxidizing conditions, the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0077] In one embodiment, the preparation method of the unsaturated carbonyl compound comprises the following steps:
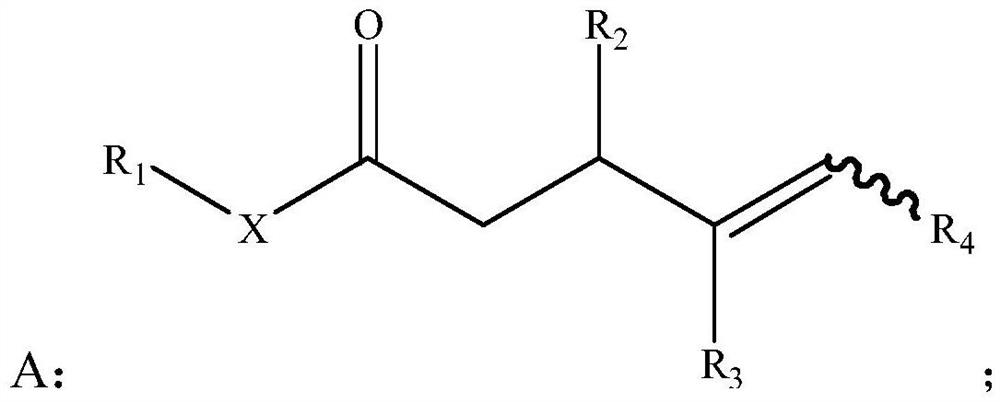
[0078] S01: Provide γ, δ-unsaturated carbonyl compound A represented by the following structural formula:
[0079]
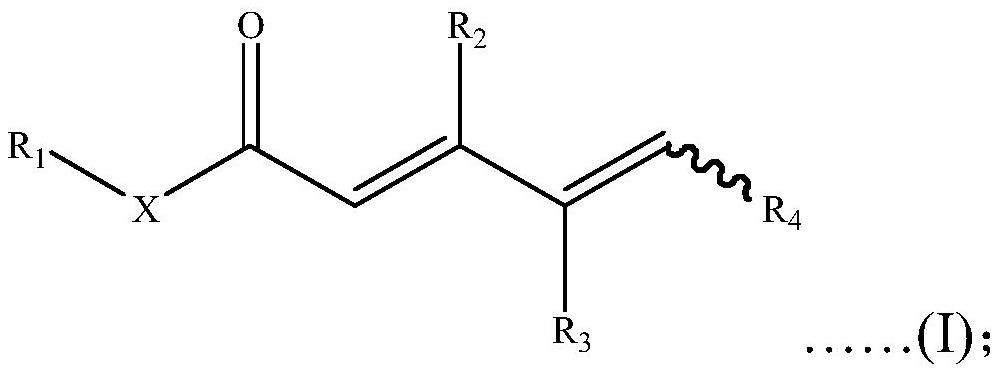
[0080] S02: Add the γ, δ-unsaturated carbonyl compound A into a reaction system containing a transition metal catalyst, additives and oxidant, and react at 10-150°C to obtain the following general structure as shown in formula (I) of unsaturated carbonyl compounds,
[0081]
[0082] Specifically, in the above step S01, R in the γ, δ-unsaturated carbonyl compound A 1 , R 2 , R 3 and R 4 For the same or different hydrogen atoms, C 1 -C 20 Alkyl, C 1 -C 20 Heteroalkyl, C 3 -C 20 Cycloalkyl, C 3 -C 20 Heterocycloalkyl, C 2 -C 20 Alkenyl, C 2 -C 20 Heteroalkenyl, C 3 -C 20 Cycloalkenyl, C 3 -C20 Heterocycloalkenyl, C 2 -C 20 Alkynyl, C 2 -C 20 Heteroalkynyl, C 3 -C 20 Cycloalkynyl, C 3 -C 20 Heterocycloalkynyl, C 1 -...
Embodiment 1
[0113] Example 1 provides (E)-N-(4-methoxybenzyl)-2,4-dienyl valeramide and its preparation method.
[0114] The structural formula of the (E)-N-(4-methoxybenzyl)-2,4-dienyl valeramide is shown in the following molecular structural formula I1:
[0115]
[0116] Its preparation steps are as follows:
[0117]Add transition metal iridium catalyst (0.002mmol, 0.02eq), silver tetrafluoroborate additive (0.005mmol, 0.05eq), copper acetate monohydrate (0.004mmol, 0.04eq), corresponding N-(4 -Methoxybenzyl)-4-valeramide substrate and 1.0 mL of anhydrous 1,4-dioxane, the reaction tube was sealed and replaced with air six times, and the resulting mixture was stirred at 60°C for 12 hours.
[0118] After the reaction was completed, the reaction solution was filtered through a glass dropper containing silica gel, rinsed with dichloromethane, and the filtrate was spin-dried to obtain the target product I1, a white solid, with a yield of >99%.
[0119] NMR characterization analysis, the...
Embodiment 2
[0121] Example 2 provides a (2E,4E)-N-(thiophene-3-methylene)-2,4-diene octanamide and a preparation method thereof.
[0122] The structural formula of the (2E,4E)-N-(thiophene-3-methylene)-2,4-diene octanamide is shown in the following molecular structural formula I2:
[0123]
[0124] Its preparation steps are as follows: Add transition metal iridium catalyst (0.002mmol, 0.02eq), silver tetrafluoroborate additive (0.005mmol, 0.05eq), copper acetate monohydrate (0.004mmol, 0.04eq), corresponding (E)-N-(thiophene-3-methylene)-4-ene octanamide substrate and 1.0 mL of anhydrous 1,4-dioxane, the reaction tube was sealed and replaced with air six times, the resulting mixture Stir at 60°C for 20 hours.
[0125] After the reaction was completed, the reaction solution was filtered through a glass dropper containing silica gel, rinsed with dichloromethane, and the filtrate was spin-dried to obtain the target product I2, a white solid, with a yield of 95%.
[0126] NMR characteriz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com