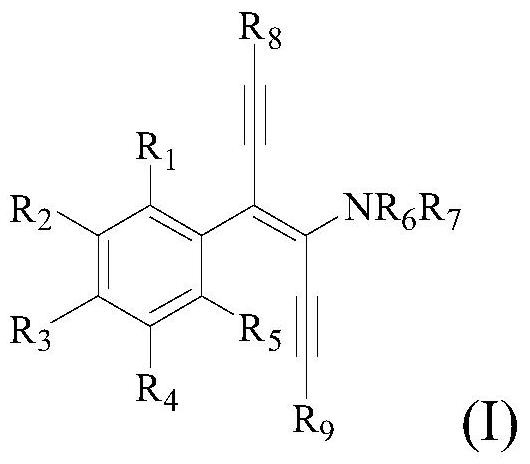
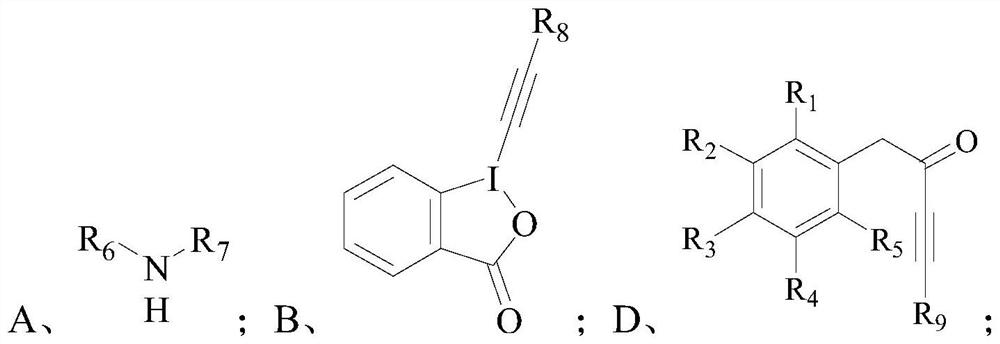
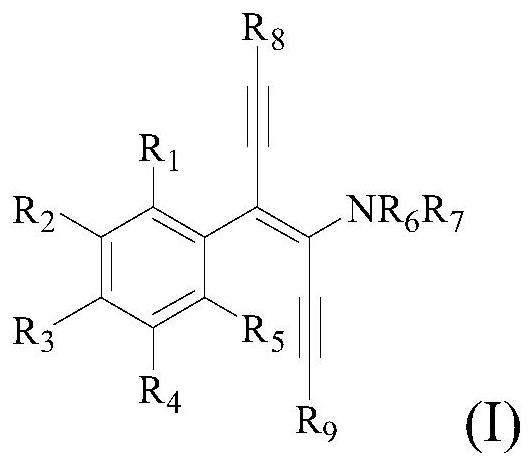
Diyne enamine compound and its preparation method and application
A technology of diacetylenic enamines and compounds is applied in the field of enamine compound synthesis, and can solve the problems of complex process, unfriendly environment, harsh preparation conditions and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0122] This embodiment provides a trans-1-(4-phenyl-1,6-bis(triisopropylsilyl)hexyl-3-en-1,5-diyne-3)pyrrolidine and its Preparation.
[0123] The structural formula of the trans-1-(4-phenyl-1,6-bis(triisopropylsilyl)hexyl-3-ene-1,5-diyne-3)pyrrolidine is shown in the following molecular structure formula I1 :
[0124]
[0125] Its preparation steps are as follows:
[0126] Add 1-phenyl-4-triisopropylsilyl-butane-3-yn-2-one (0.1mmol, 1.0eq), bipyridine (0.02mmol, 0.2eq) into a dry sealed 10mL tube, Triisopropylsilyl-alkynyl iodide ketone (0.12mmol, 1.2eq) and 2mL of anhydrous diethyl ether were added successively to gold chloride (0.01mmol, 0.1eq) and pyrrolidine (0.12mmol, 1.2eq), and placed The reaction time at 50°C is 10h.
[0127] After the reaction was completed, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a bright yellow oily liquid with a yield of 88%. Trans product: cis product>20:1.
[0128] Correlation re...
Embodiment 2
[0130] This example provides a trans-1-(4-m-methylbenzene)-1,6-bis(triisopropylsilyl)hexyl-3-ene-1,5-diyne-3)pyrrole Alkanes and their preparation methods.
[0131] The structural formula of the trans-1-(4-m-methylphenyl-1,6-bis(triisopropylsilyl)hexyl-3-ene-1,5-diyne-3)pyrrolidine is as follows molecular structural formula I2 shows:
[0132]
[0133] Its preparation method refers to the preparation of trans-1-(4-phenyl-1,6-bis(triisopropylsilyl)hexyl-3-ene-1,5-diyne-3)pyrrolidine in Example 1 method, except that 1-(3-methyl-phenyl)-4-triisopropylsilyl-butane-3-yn-2-one (0.1 mmol) was used instead of 1-phenyl-4- Triisopropylsilyl-butan-3-yn-2-one.
[0134] After the reaction was completed, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a bright yellow oily liquid with a yield of 86%. Trans product: cis product = 10.2:1.
[0135] The product I2 prepared is subjected to characterization data analysis, and the result is: ...
Embodiment 3
[0137] This example provides a trans-1-(4-p-methylphenyl-1,6-bis(triisopropylsilyl)hexyl-3-ene-1,5-diyne-3)pyrrole Alkanes and their preparation methods.
[0138] The structural formula of trans-1-(4-p-methylphenyl-1,6-bis(triisopropylsilyl)hexyl-3-en-1,5-diyne-3)pyrrolidine is as follows Molecular structure formula I3 Shown:
[0139]
[0140] Its preparation method refers to the preparation of trans-1-(4-phenyl-1,6-bis(triisopropylsilyl)hexyl-3-ene-1,5-diyne-3)pyrrolidine in Example 1 method, except that 1-(4-methyl-phenyl)-4-triisopropylsilyl-butane-3-yn-2-one (0.1 mmol) was used instead of 1-phenyl-4- Triisopropylsilyl-butan-3-yn-2-one.
[0141] After the reaction was completed, the filtrate was spin-dried and separated by column chromatography to obtain the target product as a bright yellow oily liquid with a yield of 82%. Trans product: cis product = 9.1:1.
[0142]The product I3 prepared is subjected to characterization data analysis, and the result is: 1 H NMR (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com