A drug conjugate for targeted traceless release and its preparation method and application
A conjugate and drug technology, applied in the field of medicine, can solve the problems of difficult to obtain quantitative and efficient drug loading rate, limited use of drug conjugates, high price, etc., to reduce the difficulty of preparation, reduce toxicity, and simple preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Synthesis of Ring RGDYK
[0045] The synthetic route of the ring RGDYK is shown in the route:
[0046]
[0047] Step 1, the synthesis of linear peptides
[0048] TCP resin (1 mmol / g, 2G, 2 mmol) and CH were added to a 50 ml solid phase reactor. 2 CL 2 (6 ml), swelling resin for 30 min. Pumped CH 2 CL 2 The resin was washed with anhydrous DMF (2 × 6 mL). FMOC-Gly-OH ((297 mg, 1 mmol, 0.5 eq.) Was dissolved in anhydrous DMF (4 mL), and DIEA was added to the resin (520 μL, 3 mmol, 1.5 eq.), N 2 Bubbles are mixed, and the condensation reaction is 2 h. The reaction mixture was removed, the resin was washed with DMF (4 × 6 mL), and then the resin was washed with anhydrous DMF (6 mL). A acetic acid (230 μl, 4 mmol, 2 eq.) And DMF (4 ml, 12 mmol, 6 Eq.) Of DMF (4 ml, 12 mmol, 6 Eq.) Were added to the resin, and the reaction solution was removed, the reaction solution was removed, and the resin was washed with DMF (4 × 6 mL) to give a resin. The degree of substitution ...
Embodiment 2
[0061] Example 2 Synthesis of the joint MC-VAL-CIT-PAB-CL
[0062]
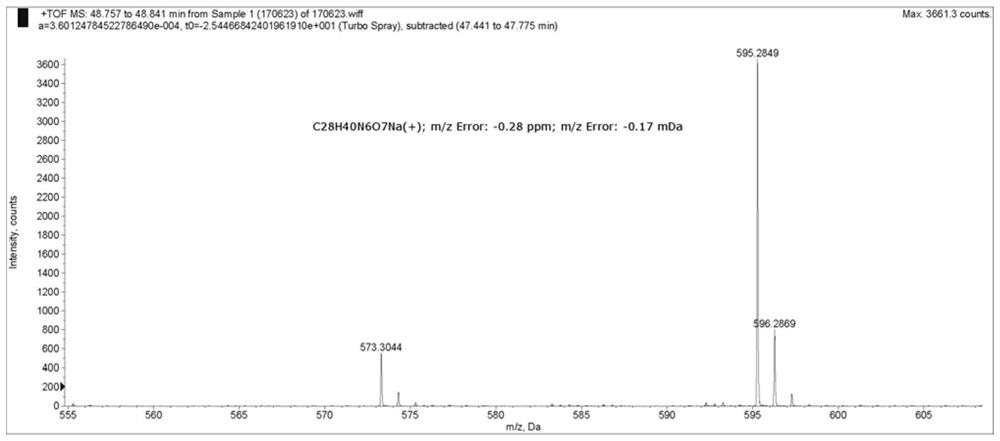
[0063] MC-VAL-CIT-PAB-Cl-Cit-Pab-Cl-Cit-Pab-OH was obtained from chlorine. Synthesis of MC-VAL-CIT-PAB-OH See Patent "Antibody Drug Conjugates, WO2014 / 191578 A1". HRMS (ESI) M / Z: Calcd FORC 28 Hide 41 N 6 O 7 [M + h] + 573.3073, Found 573.3044; Calcd for c 28 Hide 40 N 6 O 7 Na [M + NA] + 595.2856, Found595.2849.
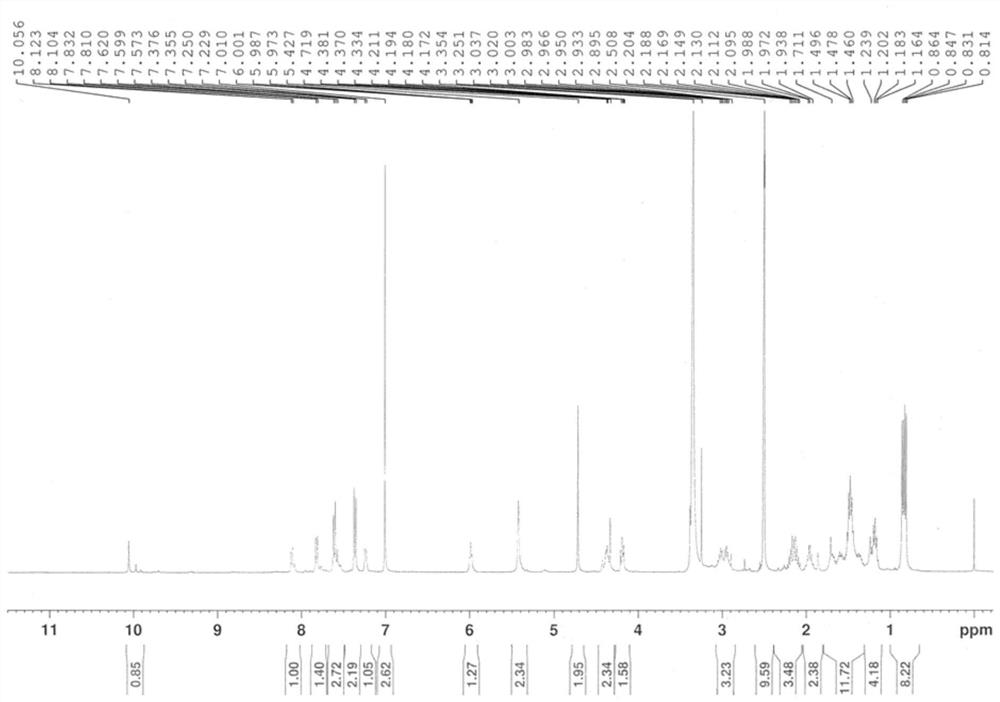
[0064] The obtained Mc-Val-Cit-Pab-OH was dissolved in anhydrous DMF, and Dichloride (2 eq.) Was added under an ice bath, and the ice bath was stirred for 2 h. The DMF was removed under reduced pressure, and the reaction product was separated by silica gel column to obtain Mc-Val-Cit-Pab-Cl. 1 H NMR (400MHz, DMSO-D6) Δ0.82 (D, J = 60Hz, 3H), 0.85 (D, J = 6.8 Hz, 3H), 1.16-1.25 (m, 2H), 1.28-1.40 (m, 1h ), 1.40-1.54 (m, 5H), 1.54-1.02 (m, 1H), 2.05-2.31 (m, 2H), 2.86-3.09 (m, 3H), 4.11-4.22 (M, 1H), 4.29-4.45 (M, 2H), 4.71 (S, 2H), 5.42 (S, 2H), 5.91-6.04 (T, J = 5.6 Hz, 1H), 7.01 (s, 2h), 7.16-7....
Embodiment 3C
[0065] Example 3COI A3 drug synthesis
[0066]
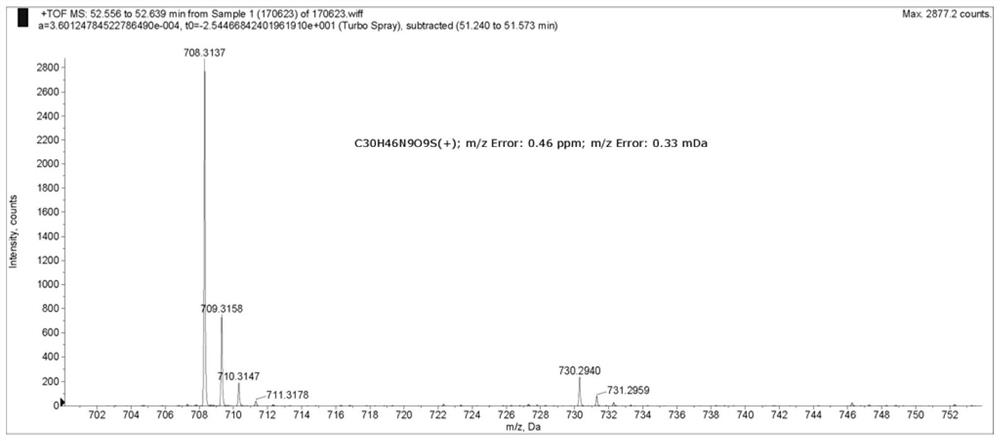
[0067]COI A3 is an analog of natural product Coibamide A, which is to replace N-Me-Ser (ME) -OH in Coibamide A with N-ME-ALA-OH, and the synthesis method see "YAO, Gy; PAN, ZY; Wu, cl; wang, w.; W.effect Synthesis and StereoChemical Revision of Coibamidea.j.am.chem. Soc., 2015, 137, 13488-13491. "HRMS (ESI) M / Z : Calcd for C 63 Hide 107 N 10 O 14 [M + h] + 1227.7968, Found 1227.7971; Calcd for c 63 Hide 106 N 10 O 14 Na [M + NA] + 1249.7788, Found1249.7751.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com