Hydroboration reaction method for aldehyde ketone catalyzed by Grignard reagent
A Grignard reagent, a technology for catalyzing aldehydes and ketones, applied in the field of hydroboration reaction, can solve the problems of long yield, high temperature and the like, and achieve the effects of high yield, low toxicity and high reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Methylmagnesium iodide catalyzes the reaction of benzaldehyde and pinacol borane, and the process is as follows:
[0016] In the glove box, sequentially add methylmagnesium iodide 0.05mol%, benzaldehyde 0.2mmol, pinacol borane 0.22mmol in the reaction bottle, then move it out of the glove box, stir for 10min, and obtain the productive rate by nuclear magnetic spectrum 99%. 1 H NMR (600MHz, CDCl 3 ): δ7.27-7.16 (m, 5H, Ar-H), 4.84 (s, 2H, OCH 2 ), 1.18(s, 12H, BOCMe 2 ). 13 C{ 1 H} NMR (151MHz, CDCl 3 ): δ139.45, 128.39, 127.47, 126.88 (Ar-C), 83.06 (BOCMe 2 ), 66.83 (OCH 2 ), 24.74 (BOCMe 2 ). 11 B{ 1 H} NMR (193MHz, CDCl 3 ): δ22.40.
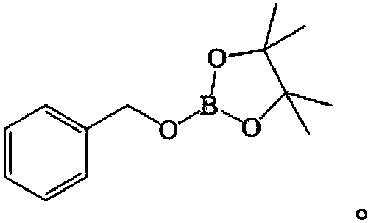
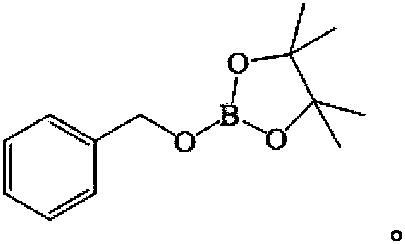
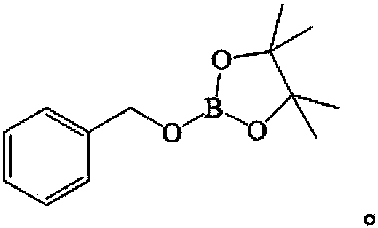
[0017] The product structure is:
[0018]
Embodiment 2
[0020] Methylmagnesium bromide catalyzes the reaction of benzaldehyde and pinacol borane, and the process is as follows:
[0021] In the glove box, sequentially add methylmagnesium bromide 0.05mol%, benzaldehyde 0.2mmol, pinacol borane 0.22mmol in the reaction bottle, then move it out of the glove box, stir for 10min, and obtain the productive rate by nuclear magnetic spectrum 98%. 1 H NMR (600MHz, CDCl 3 ): δ7.27-7.16 (m, 5H, Ar-H), 4.84 (s, 2H, OCH 2 ), 1.18(s, 12H, BOCMe 2 ). 13 C{ 1 H} NMR (151MHz, CDCl 3 ): δ139.45, 128.39, 127.47, 126.88 (Ar-C), 83.06 (BOCMe 2 ), 66.83 (OCH 2 ), 24.74 (BOCMe 2 ). 11 B{ 1 H} NMR (193MHz, CDCl 3 ): δ22.40.
[0022] The product structure is:
[0023]
Embodiment 3
[0025]Methylmagnesium chloride catalyzes the reaction of benzaldehyde and pinacol borane, and the process is as follows:
[0026] In the glove box, 0.05mol% of methylmagnesium chloride, 0.2mmol of benzaldehyde, and 0.22mmol of pinacol borane were successively added to the reaction bottle, and then it was removed from the glove box, stirred for 10min, and the yield was 97% by nuclear magnetic spectrum . 1 H NMR (600MHz, CDCl 3 ): δ7.27-7.16 (m, 5H, Ar-H), 4.84 (s, 2H, OCH 2 ), 1.18(s, 12H, BOCMe 2 ). 13 C{ 1 H} NMR (151MHz, CDCl 3 ): δ139.45, 128.39, 127.47, 126.88 (Ar-C), 83.06 (BOCMe 2 ), 66.83 (OCH 2 ), 24.74 (BOCMe 2 ). 11 B{ 1 H} NMR (193MHz, CDCl 3 ): δ22.40.
[0027] The product structure is:
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com