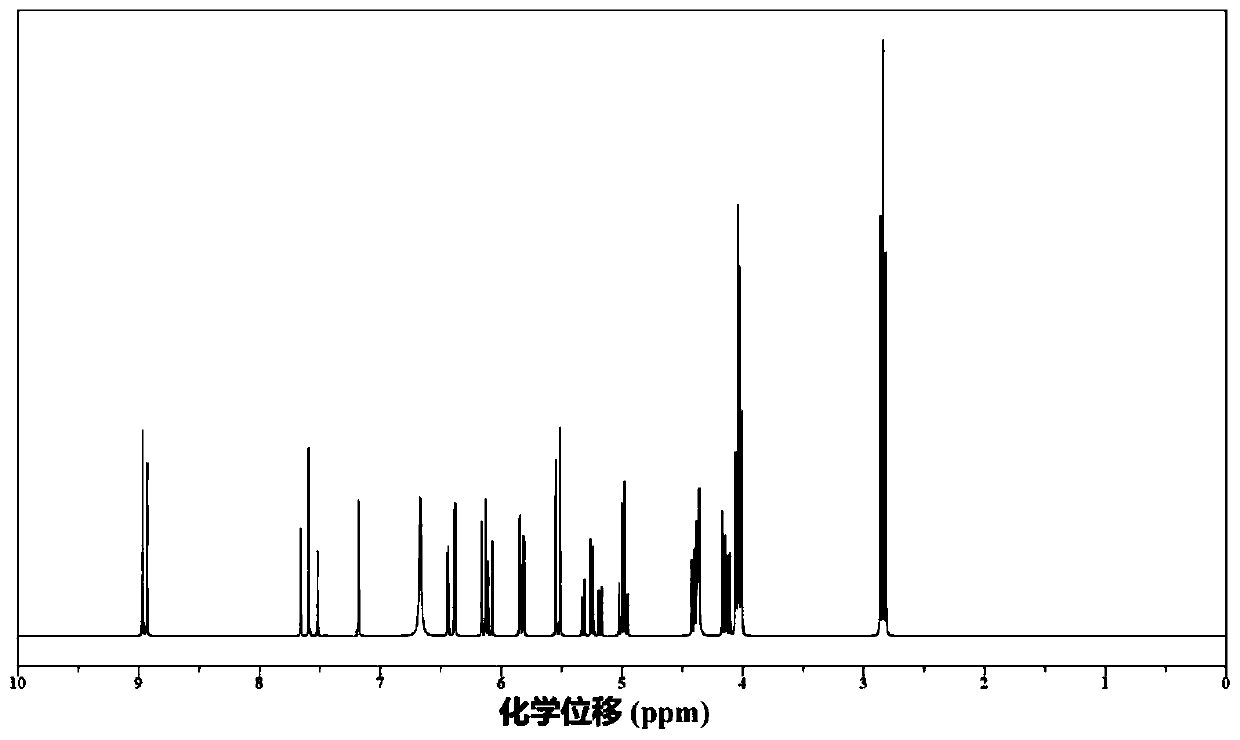
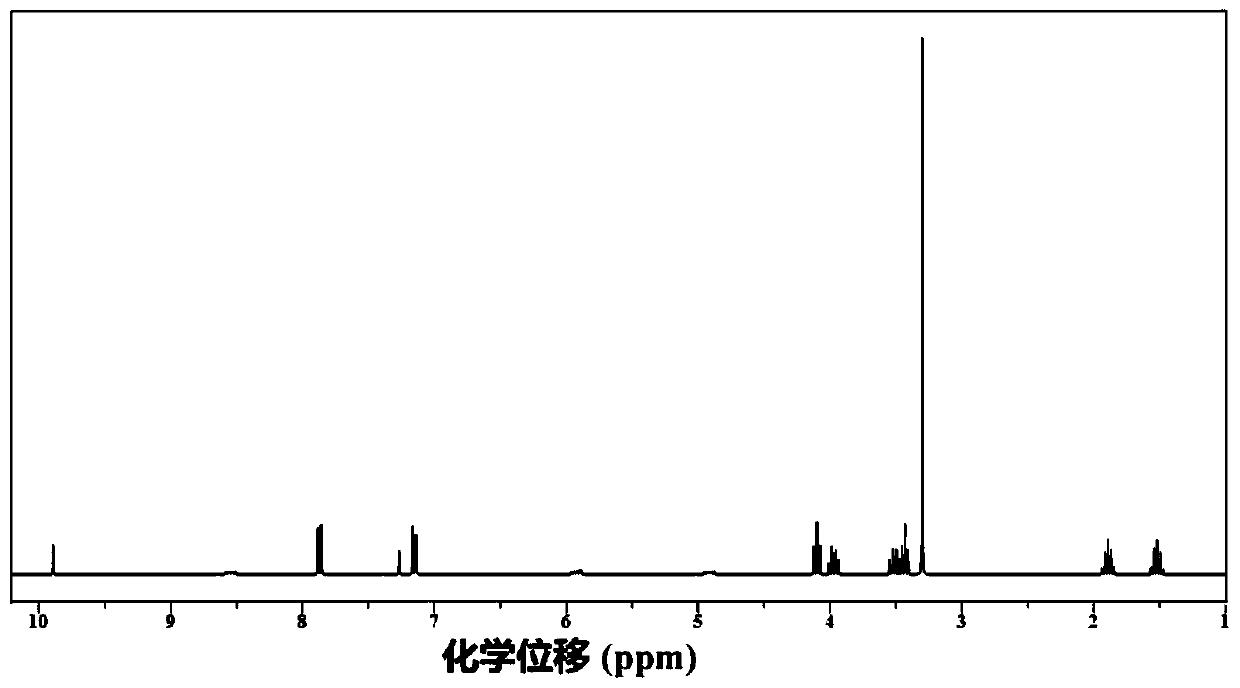
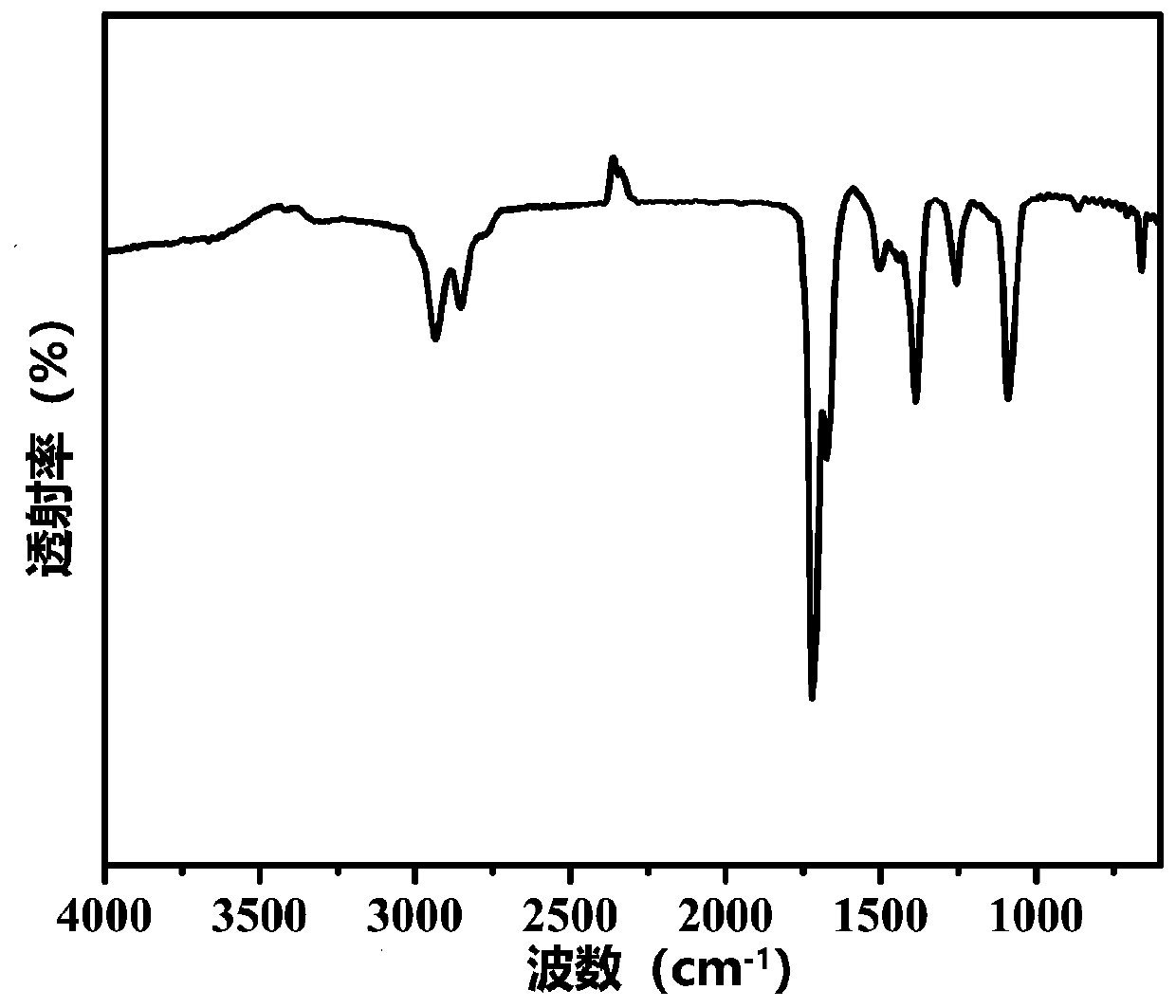
Gemcitabine prodrug compound, bionic nano medicament carrier and preparation method of carrier
A gemcitabine and biomimetic nanotechnology, applied in the field of polymers, can solve the problems of not greatly improving the chemotherapy effect of pancreatic cancer, not effectively realizing effective drug delivery and intelligent controlled release, etc., to achieve intelligent release, increase uptake, and overcome early leakage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] The preparation method of the biomimetic drug carrier, comprising:
[0050] S1. Dissolve 10 g of 2-mercaptoethanol and 0.5 g of potassium iodide in a three-necked flask filled with 30 mL of ethyl acetate, and slowly add 1 mL of hydrogen peroxide while stirring. Stirring was continued at room temperature for 2 hours. After the stirring was completed, a saturated saline solution was added, and the aqueous phase was extracted with ethyl acetate, and finally the solvent was removed by rotary evaporation to obtain 2,2'-dithiodiethanol having the structural formula (I),
[0051]
[0052] The reaction equation for S1 is,
[0053]
[0054] S2. Dissolve 5g of 2,2'-dithiodiethanol (I) and 3.9g of triethylamine prepared in S1 in dichloromethane, place in an ice-water bath, and slowly add 3.5g of propylene dropwise under stirring acid chloride. After the dropwise addition was complete, stirring was continued at room temperature for 24 hours. After the reaction is finished...
Embodiment 2
[0085] S1. Dissolve 10 g of 2-mercaptoethanol and 0.5 g of potassium iodide in a three-necked flask filled with 30 mL of ethyl acetate, and slowly add 1 mL of hydrogen peroxide while stirring. Stirring was continued at room temperature for 2 hours. After the stirring was completed, a saturated saline solution was added, and the aqueous phase was extracted with ethyl acetate, and finally the solvent was removed by rotary evaporation to obtain 2,2'-dithiodiethanol having the structural formula (I),
[0086]
[0087] The reaction equation for S1 is,
[0088]
[0089] S2. Dissolve 5g of 2,2'-dithiodiethanol (I) and 3.9g of triethylamine prepared in S1 in dichloromethane, place in an ice-water bath, and slowly add 3.5g of propylene dropwise under stirring acid chloride. After the dropwise addition was complete, stirring was continued at room temperature for 24 hours. After the reaction is finished, add a saturated aqueous common salt solution, extract therein with dichloro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com