CAR-T cell specifically targeting CD19 antigen and stably expressing PD-1 antibodies at high levels as well as application of CAR-T cell
A PD-1, chimeric antigen receptor technology, applied in the field of CART cells, can solve the problems of high drug cost, drug toxicity and side effects, and high treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
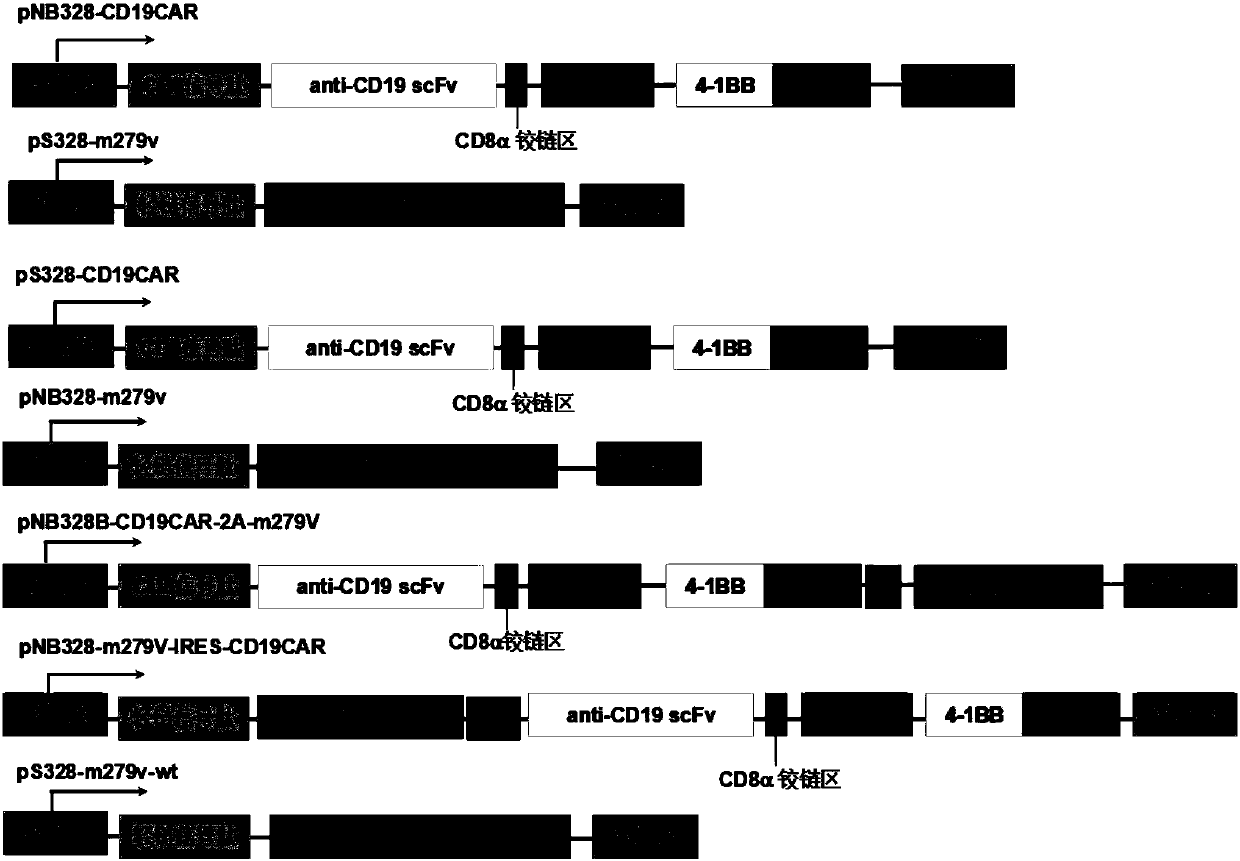
[0111] Example 1: Construction of recombinant plasmids pNB328-CD19CAR, pS328-m279V, pS328-CD19CAR, pNB328-m279V, pNB328-CD19CAR-2A-m279V, pNB328-m279V-IRES-CD19CAR and acquisition of various types of pluripotent T cells.
[0112] 1. Entrust Shanghai Jierui Biological Company to synthesize CD19CAR exogenous gene (including CD8 signal peptide, scFv, CD8 transmembrane region, CD8 hinge region, 4-1BB and CD3ζ), and introduce a polyclonal restriction site (BglII -XbaI-EcoRI-BamHI), insert a restriction site (SalI-NheI-HindIII-SpeI) downstream of it, and load it into the pNB328 vector or pS328-EF1α vector that was cut with EcoR1+SalI (the structure of pNB328 and sequence see CN 201510638974.7, which is incorporated herein by reference in its entirety; compared with pNB328, pS328 lacks the transposase coding sequence) to form recombinant plasmids, named pNB328-CD19CAR and pS328-CD19CAR, respectively.
[0113] Entrust Shanghai Jereh Biological Company to synthesize the exogenous gene ...
Embodiment 2
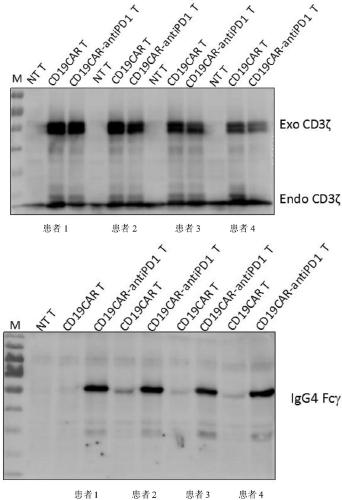
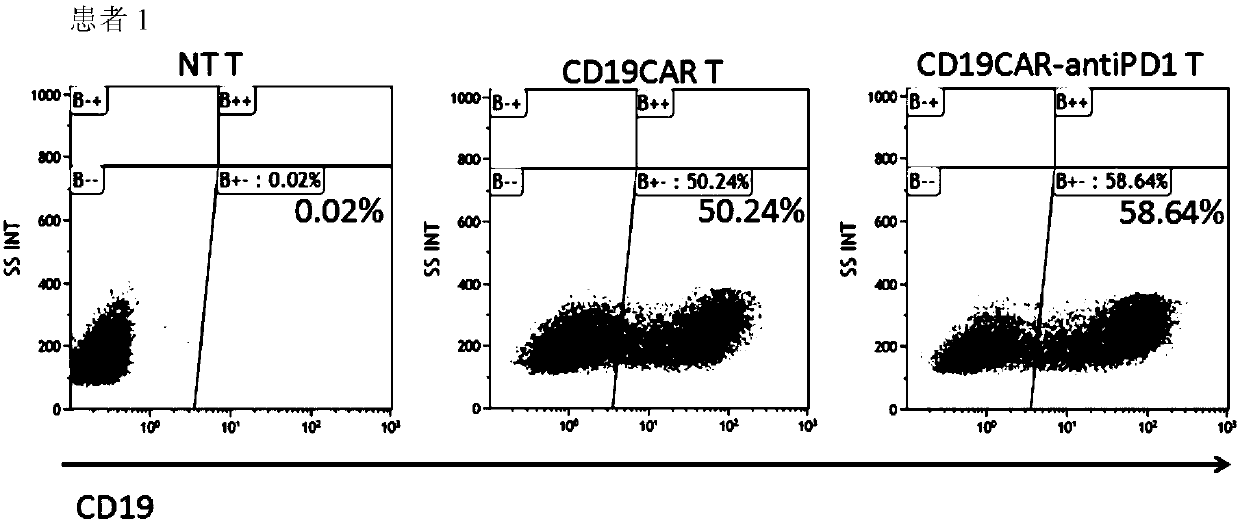
[0121] Example 2: Determination of positive rate of CD19CAR gene expression and expression of PD-1 antibody after PBMCs were modified and activated in different combinations of CD19CAR gene and PD-1 antibody gene.
[0122] Activated T cells constructed in Example 1 were divided into 2×10 6 Cell number The cells were collected and divided into 2×10 6 Cells / well were spread in a 6-well plate with 3ml of AIM-V medium, cultured in a 5% CO2 incubator at 37°C, and the cell supernatant was collected after 24 hours of culture, and stored at -20°C for future use.
[0123] Double-antibody sandwich ELISA method (using human PD-1 recombinant protein coated microtiter plate, HRP-labeled mouse anti-human IgG4 mAb) was used to detect, commercialized PD-1 antibody was used as a standard, and the sample to be tested was 5 times After dilution, the expression level of PD-1 antibody in the genetically modified T cells was quantitatively detected. Collect 1×10 at the same time 6 Cell pellet, w...
Embodiment 3
[0128] Example 3: Determination of the positive rate of CD19CAR gene expression and the expression of PD-1 antibody in PBMCs modified with different mass ratios of pNB328-CD19CAR and pS328-m279V plasmids.
[0129] The recombinant plasmids pNB328-CD19CAR and pS328-m279V constructed in Example 1 were electroporated into PBMCs cells (1:1, 3:5, 1:3, 1:7) at different mass ratios to obtain multiple simultaneous expression of CD19CAR gene and PD -1 antibody T cells. These T cells were divided into 2 × 10 6 Cell number The cells were collected and divided into 2×10 6 Cells / well were spread in a 6-well plate with 3ml of AIM-V medium, cultured in a 5% CO2 incubator at 37°C, and the cell supernatant was collected after 24 hours of culture, and stored at -20°C for future use.
[0130] Double-antibody sandwich ELISA method (using human PD-1 recombinant protein coated microtiter plate, HRP-labeled mouse anti-human IgG4 mAb) was used to detect, commercialized PD-1 antibody was used as a s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com